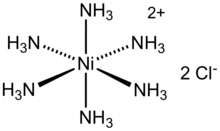
Hexaamminenickel chloride
| |

| |
| Names | |
|---|---|
| IUPAC name Hexaamminenickel(II) chloride | |
| Other names Nickel hexammine chloride, hexamminenickel chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.149.740 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cl2H18N6Ni | |
| Molar mass | 231.78 g·mol−1 |
| Appearance | violet solid |
| Density | 1.51 g/cm3 |
| Melting point | decomposes |
| Solubility | soluble in NH3 |
| Structure | |
| octahedral | |
| 0 D | |
| Related compounds | |
Other cations |
[Cr(NH3)6]Cl3 [Co(NH3)6]Cl3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Hexaamminenickel chloride is the chemical compound with the formula [Ni(NH3)6]Cl2. It is the chloride salt of the metal ammine complex [Ni(NH3)6]2+. The cation features six ammonia (called ammines in coordination chemistry) ligands attached to the nickel(II) ion.[1]
Properties and structure
[Ni(NH3)6]2+, like all octahedral nickel(II) complexes, is paramagnetic with two unpaired electrons localized on each Ni center. [Ni(NH3)6]Cl2 is prepared by treating aqueous nickel(II) chloride with ammonia. It is useful as a molecular source of anhydrous nickel(II).[2]
Related compounds
One commercial method for extraction of nickel from its sulfide ores involves the sulfate salt of [Ni(NH3)6]2+. In this process, the partially purified ore is treated with air and ammonia as described with this simplified equation:[3]
- NiS + 2 O2 + 6 NH3 → [Ni(NH3)6]SO4
References
- ^ Eßmann, Ralf; Kreiner, Guido; Niemann, Anke; Rechenbach, Dirk; Schmieding, Axel; Sichla, Thomas; Zachwieja, Uwe; Jacobs, Herbert (1996). "Isotype Strukturen einiger Hexaamminmetall(II)‐halogenide von 3d‐Metallen: [V(NH3)6]I2, [Cr(NH3)6]I2, [Mn(NH3)6]Cl2, [Fe(NH3)6]Cl2, [Fe(NH3)6]Br2, [Co(NH3)6]Br2, und [Ni(NH3)6]Cl2". Zeitschrift für anorganische und allgemeine Chemie. 622 (7): 1161–1166. doi:10.1002/zaac.19966220709.
- ^ G. S. Girolami, T. B. Rauchfuss, and R. J. Angelici (1999) Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA.ISBN 0935702482
- ^ Kerfoot, Derek G. E. (2000). "Nickel". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_157. ISBN 978-3-527-30385-4.
