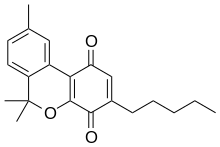
HU-345
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H24O3 |
| Molar mass | 324.420 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
HU-345 (cannabinol quinone) is a drug that is able to inhibit aortic ring angiogenesis more potently than its parent compound cannabinol (CBN).[1][2] It exhibits no psychoactive effects on the body.
HU-345 can be derived through the oxidative degradation of CBN.[3]
See also
References
- ^ Kogan NM, Blázquez C, Alvarez L, Gallily R, Schlesinger M, Guzmán M, Mechoulam R (July 2006). "A cannabinoid quinone inhibits angiogenesis by targeting vascular endothelial cells". Molecular Pharmacology. 70 (1): 51–59. doi:10.1124/mol.105.021089. PMID 16571653. S2CID 4830577.
- ^ US patent 0092584, Mechoulam R, Kogan NM, Rabinowitz R, Schlesinger M, "Therapeutic Use of Quinonoid Derivatives of Cannabinoids", granted 2011-04-21
- ^ Kogan NM, Peters M, Mechoulam R (March 2021). "Cannabinoid Quinones-A Review and Novel Observations". Molecules. 26 (6): 1761. doi:10.3390/molecules26061761. PMC 8003933. PMID 33801057.
