Epoxide

In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.[1]
Nomenclature
A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound 1,2-epoxyheptane, which can also be called 1,2-heptene oxide.
A polymer formed from epoxide precursors is called an epoxy. However, few if any of the epoxy groups in the resin survive the curing process.
Synthesis
The dominant epoxides industrially are ethylene oxide and propylene oxide, which are produced respectively on the scales of approximately 15 and 3 million tonnes/year.[2]
Aside from ethylene oxide, most epoxides are generated when peroxidized reagents donate a single oxygen atom to an alkene. Safety considerations weigh on these reactions because organic peroxides are prone to spontaneous decomposition or even combustion.
Both t-butyl hydroperoxide and ethylbenzene hydroperoxide can be used as oxygen sources during propylene oxidation (although a catalyst is required as well, and most industrial producers use dehydrochlorination instead).[3]
Ethylene oxidation
The ethylene oxide industry generates its product from reaction of ethylene and oxygen. Modified heterogeneous silver catalysts are typically employed.[4] According to a reaction mechanism suggested in 1974[5] at least one ethylene molecule is totally oxidized for every six that are converted to ethylene oxide:
Only ethylene produces an epoxide during incomplete combustion. Other alkenes fail to react usefully, even propylene, though TS-1 supported Au catalysts can selectively epoxidize propylene.[6]
Organic peroxides and metal catalysts
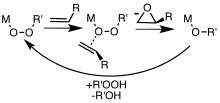
Metal complexes are useful catalysts for epoxidations involving hydrogen peroxide and alkyl hydroperoxides. Metal-catalyzed epoxidations were first explored using tert-butyl hydroperoxide (TBHP).[7] Association of TBHP with the metal (M) generates the active metal peroxy complex containing the MOOR group, which then transfers an O center to the alkene.[8]
Vanadium(II) oxide catalyzes the epoxidation at specifically less-substituted alkenes.[9]
Nucleophilic epoxidation
Electron-deficient olefins, such as enones and acryl derivatives can be epoxidized using nucleophilic oxygen compounds such as peroxides. The reaction is a two-step mechanism. First the oxygen performs a nucleophilic conjugate addition to give a stabilized carbanion. This carbanion then attacks the same oxygen atom, displacing a leaving group from it, to close the epoxide ring.
Transfer from peroxycarboxylic acids
Peroxycarboxylic acids, which are more electrophilic than other peroxides, convert alkenes to epoxides without the intervention of metal catalysts. In specialized applications, dioxirane reagents (e.g. dimethyldioxirane) perform similarly, but are more explosive.
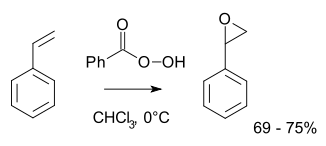
Typical laboratory operations employ the Prilezhaev reaction.[10][11] This approach involves the oxidation of the alkene with a peroxyacid such as mCPBA. Illustrative is the epoxidation of styrene with perbenzoic acid to styrene oxide:[12]
The stereochemistry of the reaction is quite sensitive. Depending on the mechanism of the reaction and the geometry of the alkene starting material, cis and/or trans epoxide diastereomers may be formed. In addition, if there are other stereocenters present in the starting material, they can influence the stereochemistry of the epoxidation.
The reaction proceeds via what is commonly known as the "Butterfly Mechanism".[13] The peroxide is viewed as an electrophile, and the alkene a nucleophile. The reaction is considered to be concerted. The butterfly mechanism allows ideal positioning of the O−O sigma star orbital for C−C π electrons to attack.[14] Because two bonds are broken and formed to the epoxide oxygen, this is formally an example of a coarctate transition state.
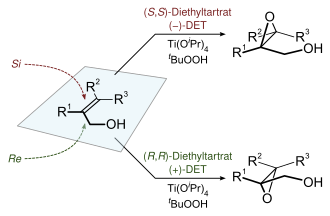
Asymmetric epoxidations
Chiral epoxides can often be derived enantioselectively from prochiral alkenes. Many metal complexes give active catalysts, but the most important involve titanium, vanadium, and molybdenum.[15][16]
Hydroperoxides are also employed in catalytic enantioselective epoxidations, such as the Sharpless epoxidation and the Jacobsen epoxidation. Together with the Shi epoxidation, these reactions are useful for the enantioselective synthesis of chiral epoxides. Oxaziridine reagents may also be used to generate epoxides from alkenes.
The Sharpless epoxidation reaction is one of the premier enantioselective chemical reactions. It is used to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols.[17][18]
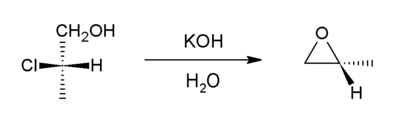
Dehydrohalogenation and other γ eliminations

Halohydrins react with base to give epoxides.[20] The reaction is spontaneous because the energetic cost of introducing the ring strain (13 kcal/mol) is offset by the larger bond enthalpy of the newly introduced C-O bond (when compared to that of the cleaved C-halogen bond).
Formation of epoxides from secondary halohydrins is predicted to occur faster than from primary halohydrins due to increased entropic effects in the secondary halohydrin, and tertiary halohydrins react (if at all) extremely slowly due to steric crowding. [21]
Starting with propylene chlorohydrin, most of the world's supply of propylene oxide arises via this route.[3]
An intramolecular epoxide formation reaction is one of the key steps in the Darzens reaction.
In the Johnson–Corey–Chaykovsky reaction epoxides are generated from carbonyl groups and sulfonium ylides. In this reaction, a sulfonium is the leaving group instead of chloride.
Biosynthesis
Epoxides are uncommon in nature. They arise usually via oxygenation of alkenes by the action of cytochrome P450.[22] (but see also the short-lived epoxyeicosatrienoic acids which act as signalling molecules.[23] and similar epoxydocosapentaenoic acids, and epoxyeicosatetraenoic acids.)
Arene oxides are intermediates in the oxidation of arenes by cytochrome P450. For prochiral arenes (naphthalene, toluene, benzoates, benzopyrene), the epoxides are often obtained in high enantioselectivity.
Reactions
Ring-opening reactions dominate the reactivity of epoxides.
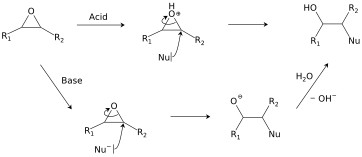
Hydrolysis and addition of nucleophiles
Epoxides react with a broad range of nucleophiles, for example, alcohols, water, amines, thiols, and even halides. With two often-nearly-equivalent sites of attack, epoxides exemplify "ambident substrates".[24] Ring-opening regioselectivity in asymmetric epoxides generally follows the SN2 pattern of attack at the least-substituted carbon,[25] but can be affected by carbocation stability under acidic conditions.[26] This class of reactions is the basis of epoxy glues and the production of glycols.[19]
Lithium aluminium hydride or aluminium hydride both reduce epoxides through a simple nucleophilic addition of hydride (H−); they produce the corresponding alcohol.[27]
Polymerization and oligomerization
Polymerization of epoxides gives polyethers. For example ethylene oxide polymerizes to give polyethylene glycol, also known as polyethylene oxide. The reaction of an alcohol or a phenol with ethylene oxide, ethoxylation, is widely used to produce surfactants:[28]
- ROH + n C2H4O → R(OC2H4)nOH
With anhydrides, epoxides give polyesters.[29]
Metallation and deoxygenation
Lithiation cleaves the ring to β-lithioalkoxides.[30]
Epoxides can be deoxygenated using oxophilic reagents, with loss or retention of configuration.[31] The combination of tungsten hexachloride and n-butyllithium gives the alkene.[32][33]
When treated with thiourea, epoxides convert to the episulfide (thiiranes).
Other reactions
- Epoxides undergo ring expansion reactions, illustrated by the insertion of carbon dioxide to give cyclic carbonates.
- An epoxide adjacent to an alcohol can undergo the Payne rearrangement in base.
Uses
- Bisphenol A diglycidyl ether is a component in common household "epoxy".
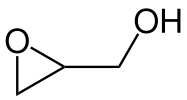
- The chemical structure of the epoxide glycidol, a common chemical intermediate.
- Epothilones are naturally occurring epoxides.
- 3,4-Epoxycyclohexylmethyl-3',4'-epoxycyclohexane carboxylate, precursor to coatings.[34]
- Epoxidized linolein, a major component of epoxidized soybean oil (ESBO), a commercially important plasticizer.
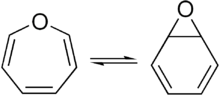
- Benzene oxide exists in equilibrium with the oxepin isomer.
Ethylene oxide is widely used to generate detergents and surfactants by ethoxylation. Its hydrolysis affords ethylene glycol. It is also used for sterilisation of medical instruments and materials.
The reaction of epoxides with amines is the basis for the formation of epoxy glues and structural materials. A typical amine-hardener is triethylenetetramine (TETA).
Safety
Epoxides are alkylating agents, making many of them highly toxic.[35]
See also
Further reading
- Massingill, J. L.; Bauer, R. S. (2000-01-01). "Epoxy Resins". In Craver, Clara D.; Carraher, Charles E. (eds.). Applied Polymer Science: 21st Century. Oxford: Pergamon. pp. 393–424. doi:10.1016/b978-008043417-9/50023-4. ISBN 978-0-08-043417-9. Retrieved 2023-12-20.
References
- ^ Guenter Sienel; Robert Rieth; Kenneth T. Rowbottom. "Epoxides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_531. ISBN 978-3527306732.
- ^ Siegfried Rebsdat; Dieter Mayer. "Ethylene Oxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_117. ISBN 978-3527306732.
- ^ a b Dietmar Kahlich, Uwe Wiechern, Jörg Lindner "Propylene Oxide" in Ullmann's Encyclopedia of Industrial Chemistry, 2002 by Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_239
- ^ Sajkowski, D. J.; Boudart, M. (1987). "Structure Sensitivity of the Catalytic Oxidation of Ethene by Silver". Catalysis Reviews. 29 (4): 325–360. doi:10.1080/01614948708078611.
- ^ Kilty P. A.; Sachtler W. M. H. (1974). "The mechanism of the selective oxidation of ethylene to ethylene oxide". Catalysis Reviews: Science and Engineering. 10: 1–16. doi:10.1080/01614947408079624.
- ^ Nijhuis, T. Alexander; Makkee, Michiel; Moulijn, Jacob A.; Weckhuysen, Bert M. (1 May 2006). "The Production of Propene Oxide: Catalytic Processes and Recent Developments". Industrial & Engineering Chemistry Research. 45 (10): 3447–3459. doi:10.1021/ie0513090. hdl:1874/20149. S2CID 94240406.
- ^ Indictor N., Brill W. F. (1965). "Metal Acetylacetonate Catalyzed Epoxidation of Olefins with t-Butyl Hydroperoxide". J. Org. Chem. 30 (6): 2074. doi:10.1021/jo01017a520.
- ^ Thiel W. R. (1997). "Metal catalyzed oxidations. Part 5. Catalytic olefin epoxidation with seven-coordinate oxobisperoxo molybdenum complexes: a mechanistic study". Journal of Molecular Catalysis A: Chemical. 117: 449–454. doi:10.1016/S1381-1169(96)00291-9.
- ^ Taber, Douglass (25 Sep 2006). "Selective reactions of Alkenes". Organic Chemistry Highlights.
- ^ March, Jerry. 1985. Advanced Organic Chemistry, Reactions, Mechanisms and Structure. 3rd ed. John Wiley & Sons. ISBN 0-471-85472-7.
- ^ Nikolaus Prileschajew (1909). "Oxydation ungesättigter Verbindungen mittels organischer Superoxyde" [Oxidation of unsaturated compounds by means of organic peroxides]. Berichte der Deutschen Chemischen Gesellschaft (in German). 42 (4): 4811–4815. doi:10.1002/cber.190904204100.
- ^ Harold Hibbert and Pauline Burt (1941). "Styrene Oxide". Organic Syntheses; Collected Volumes, vol. 1, p. 494.
- ^ Paul D. Bartlett (1950). "Recent work on the mechanisms of peroxide reactions". Record of Chemical Progress. 11: 47–51.
- ^ John O. Edwards (1962). Peroxide Reaction Mechanisms. Interscience, New York. pp. 67–106.
- ^ Berrisford, D. J.; Bolm, C.; Sharpless, K. B. (2003). "Ligand-Accelerated Catalysis". Angew. Chem. Int. Ed. Engl. 95 (10): 1059–1070. doi:10.1002/anie.199510591.
- ^ Sheldon R. A. (1980). "Synthetic and mechanistic aspects of metal-catalysed epoxidations with hydroperoxides". Journal of Molecular Catalysis. 1: 107–206. doi:10.1016/0304-5102(80)85010-3.
- ^ Katsuki, T.; Sharpless, K. B. (1980). "The first practical method for asymmetric epoxidation". J. Am. Chem. Soc. 102 (18): 5974–5976. doi:10.1021/ja00538a077.
- ^ Hill, J. G.; Sharpless, K. B.; Exon, C. M.; Regenye, R. Org. Synth., Coll. Vol. 7, p. 461 (1990); Vol. 63, p. 66 (1985). (Article Archived 2013-09-27 at the Wayback Machine)
- ^ a b Pham, Ha Q.; Marks, Maurice J. (2005). "Epoxy Resins". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a09_547.pub2. ISBN 978-3527306732.
- ^ Koppenhoefer, B.; Schurig, V. (1993). "(R)-Alkyloxiranes of High Enantiomeric Purity from (S)-2-Chloroalkanoic Acids via (S)-2-Chloro-1-Alkanols: (R)-Methyloxirane". Organic Syntheses; Collected Volumes, vol. 8, p. 434.
- ^ Silva, P.J. (2023). "Computational insights into the spontaneity of epoxide formation from halohydrins and other mechanistic details of Williamson's ether synthesis". Chem. J. Mold. 18 (2): 87–95. doi:10.19261/cjm.2023.1083.
- ^ Thibodeaux C. J. (2012). "Enzymatic Chemistry of Cyclopropane, Epoxide, and Aziridine Biosynthesis". Chem. Rev. 112 (3): 1681–1709. doi:10.1021/cr200073d. PMC 3288687. PMID 22017381.
- ^ Boron WF (2003). Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. p. 108. ISBN 978-1-4160-2328-9.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 517, ISBN 978-0-471-72091-1
- ^ Warren, Stuart; Wyatt, Paul (2008). Organic Synthesis: the disconnection approach (2nd ed.). Wiley. p. 39.
- ^ Rzepa, Henry (28 April 2013). "How to predict the regioselectivity of epoxide ring opening". Chemistry with a twist.
- ^ Bruce Rickborn and Wallace E. Lamke (1967). "Reduction of epoxides. II. The lithium aluminum hydride and mixed hydride reduction of 3-methylcyclohexene oxide". J. Org. Chem. 32 (3): 537–539. doi:10.1021/jo01278a005.
- ^ Kosswig, Kurt (2002). "Surfactants". In Elvers, Barbara; et al. (eds.). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, GER: Wiley-VCH. doi:10.1002/14356007.a25_747. ISBN 978-3527306732.
- ^ Julie M. Longo; Maria J. Sanford; Geoffrey W. Coates (2016). "Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure–Property Relationships". Chem. Rev. 116 (24): 15167–15197. doi:10.1021/acs.chemrev.6b00553. PMID 27936619.
- ^ B. Mudryk; T. Cohen (1995). "1,3-Diols From Lithium Β-lithioalkoxides Generated By The Reductive Lithiation Of Epoxides: 2,5-dimethyl-2,4-hexanediol". Org. Synth. 72: 173. doi:10.15227/orgsyn.072.0173.
- ^ Takuya Nakagiri; Masahito Murai; Kazuhiko Takai (2015). "Stereospecific Deoxygenation of Aliphatic Epoxides to Alkenes under Rhenium Catalysis". Org. Lett. 17 (13): 3346–9. doi:10.1021/acs.orglett.5b01583. PMID 26065934.
- ^ K. Barry Sharpless, Martha A. Umbreit (1981). "Deoxygenation of Epoxides with Lower Valent Tungsten Halides: trans-Cyclododecene". Org. Synth. 60: 29. doi:10.15227/orgsyn.060.0029.
- ^ K. Barry Sharpless; Martha A. Umbreit; Marjorie T. Nieh; Thomas C. Flood (1972). "Lower valent tungsten halides. New class of reagents for deoxygenation of organic molecules". J. Am. Chem. Soc. 94 (18): 6538–6540. doi:10.1021/ja00773a045.
- ^ Sasaki, Hiroshi (February 2007). "Curing properties of cycloaliphatic epoxy derivatives". Progress in Organic Coatings. 58 (2–3): 227–230. doi:10.1016/j.porgcoat.2006.09.030.
- ^ Niederer, Christian; Behra, Renata; Harder, Angela; Schwarzenbach, René P.; Escher, Beate I. (2004). "Mechanistic approaches for evaluating the toxicity of reactive organochlorines and epoxides in green algae". Environmental Toxicology and Chemistry. 23 (3): 697–704. doi:10.1897/03-83. PMID 15285364. S2CID 847639.





![3,4-Epoxycyclohexylmethyl-3',4'-epoxycyclohexane carboxylate, precursor to coatings.[34]](https://upload.wikimedia.org/wikipedia/commons/thumb/0/0e/Diepoxyester.svg/175px-Diepoxyester.svg.png)