Delavirdine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rescriptor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600034 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 98% |
| Metabolism | Liver (CYP3A4- and CYP2D6-mediated) |
| Elimination half-life | 5.8 hours |
| Excretion | Kidney (51%) and feces (44%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
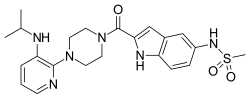
| Formula | C22H28N6O3S |
| Molar mass | 456.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Delavirdine (DLV) (brand name Rescriptor) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) marketed by ViiV Healthcare. It is used as part of highly active antiretroviral therapy (HAART) for the treatment of human immunodeficiency virus (HIV) type 1. It is presented as the mesylate. The recommended dosage is 400 mg, three times a day.
Although delavirdine was approved by the U.S. Food and Drug Administration in 1997, its efficacy is lower than other NNRTIs, especially efavirenz, and it also has an inconvenient schedule. These factors have led the U.S. DHHS not to recommend its use as part of initial therapy.[1] The risk of cross-resistance across the NNRTI class, as well as its complex set of drug interactions, makes the place of delavirdine in second-line and salvage therapy unclear, and it is currently rarely used.
Its manufacturing and distribution was discontinued in the United States and Canada.[2][3][4]
Interactions
Like ritonavir, delavirdine is an inhibitor of cytochrome P450 isozyme CYP3A4, and interacts with many medications. It should not be administered with a wide range of drugs, including amprenavir, fosamprenavir, simvastatin, lovastatin, rifampin, rifabutin, rifapentine, St John's wort, midazolam, triazolam, ergot medications, and several medications for acid reflux.[1]
Adverse effects
The most common adverse event is moderate to severe rash, which occurs in up to 20% of patients.[5] Other common adverse events include fatigue, headache and nausea. Liver toxicity has also been reported.
Synthesis
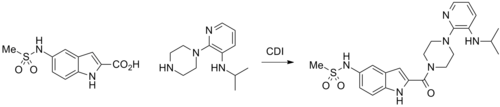
Modification of the scheme that was done for ateviridine q.v. by performing the reductive alkylation with acetone gives 2 after removal of the protecting group. Acylation of this amine with the imidazolide from 5-Methylsulfonaminoindole-2-carboxylic acid (1) affords the amide, reverse transcriptase inhibitor, atevirdine.
References
- ^ a b DHHS panel. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents (May 4, 2006). (Available for download from AIDSInfo Archived 2006-05-06 at the Wayback Machine)
- ^ RPh, Diana Ernst (11 May 2017). "HIV Drug Soon to Be Unavailable". MPR.
- ^ "Delavirdine: Indications, Side Effects, Warnings". Drugs.com.
- ^ "Prescription drug RESCRIPTOR (delavirdine mesylate) – DrugFinder.ca". drugfinder.ca.
- ^ "RESCRIPTOR brand of delavirdine mesylate tablets. Product information" (PDF). Archived from the original (PDF) on 2006-04-15. Retrieved 2006-05-18.
- ^ WO 9109849, Romero DL, Mitchell MA, Thomas RC, Palmer JR, Tarpley WG, Aristoff PA, Smith HW, "Diaromatic Substituted Anti-AIDS Compounds", published 11 July 1991, assigned to Upjohn Company
- ^ US 5563142, Palmer JR, Romero DL, Aristoff PA, Thomas RC, Smith HW, "Diaromatic substituted compounds as anti-HIV-1 agents", published 8 October 1996, assigned to Upjohn Company
- ^ Romero DL, Morge RA, Genin MJ, Biles C, Busso M, Resnick L, et al. (May 1993). "Bis(heteroaryl)piperazine (BHAP) reverse transcriptase inhibitors: structure-activity relationships of novel substituted indole analogues and the identification of 1-[(5-methanesulfonamido-1H-indol-2-yl)-carbonyl]-4-[3- [(1-methylethyl)amino]-pyridinyl]piperazine monomethanesulfonate (U-90152S), a second-generation clinical candidate". Journal of Medicinal Chemistry. 36 (10): 1505–8. doi:10.1021/jm00062a027. PMID 7684450.
External links
- "Delavirdine". Drug Information Portal. U.S. National Library of Medicine.
