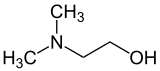
Dimethylethanolamine
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-(Dimethylamino)ethan-1-ol | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | DMAE, DMEA |
| 1209235 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.221 |
| EC Number |
|
| KEGG | |
| MeSH | Deanol |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2051 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H11NO | |
| Molar mass | 89.138 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Fishy, ammoniacal |
| Density | 890 mg mL−1 |
| Melting point | −59.00 °C; −74.20 °F; 214.15 K |
| Boiling point | 134.1 °C; 273.3 °F; 407.2 K |
| log P | −0.25 |
| Vapor pressure | 816 Pa (at 20 °C) |
| Acidity (pKa) | 9.23 (at 20 °C)[1] |
| Basicity (pKb) | 4.77 (at 20 °C) |
Refractive index (nD) |
1.4294 |
| Pharmacology | |
| N06BX04 (WHO) | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H226, H302, H312, H314, H332 | |
| P280, P305+P351+P338, P310 | |
| Flash point | 39 °C (102 °F; 312 K) |
| Explosive limits | 1.4–12.2% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| Related compounds | |
Related alkanols |
|
Related compounds |
Diethylhydroxylamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dimethylethanolamine (DMAE or DMEA) is an organic compound with the formula (CH3)2NCH2CH2OH. It is bifunctional, containing both a tertiary amine and primary alcohol functional groups. It is a colorless viscous liquid. It is used in skin care products for improving skin tone and also taken orally as a nootropic. It is prepared by the ethoxylation of dimethylamine.[2]
Industrial uses
Dimethylaminoethanol is used as a curing agent for polyurethanes and epoxy resins. It is a precursor to other chemicals, such as the nitrogen mustard 2-dimethylaminoethyl chloride.[3] The acrylate ester, dimethylaminoethyl acrylate is used as a flocculating agent.[4]
Related compounds are used in gas purification, e.g. removal of hydrogen sulfide from sour gas streams.
Human uses
The bitartrate salt of DMAE, i.e. N,N-dimethylethanolamine bitartrate, is sold as a dietary supplement.[5] It is a white powder providing 37% DMAE.[6]
Animal tests show possible benefit for improving spatial memory[7] and working memory.[8]
See also
- Choline
- Diphenhydramine
- Doxylamine
- Ethanolamine
- Meclofenoxate (Centrophenoxine)
- Orphenadrine
References
- ^ Littel, RJ; Bos, M; Knoop, GJ (1990). "Dissociation constants of some alkanolamines at 293, 303, 318, and 333 K" (PDF). Journal of Chemical and Engineering Data. 35 (3): 276–77. doi:10.1021/je00061a014. INIST 19352048.
- ^ Matthias Frauenkron; Johann-Peter Melder; Günther Ruider; Roland Rossbacher; Hartmut Höke (2002). "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001. ISBN 978-3-527-30673-2.
- ^ Ashford, Robert D. (2011). Ashford's Dictionary of Industrial Chemicals, 3rd edition. Wavelength. p. 3294. ISBN 9780952267430. OCLC 863579691. Retrieved 2023-10-04.
{{cite book}}:|website=ignored (help) - ^ "Dimethylaminoethyl Acrylate - Global Review 2020 to 2030". Fact.MR. Retrieved 2023-10-04.
- ^ Haneke, Karen E.; Masten, Scott (November 2002). Dimethylethanolamine (DMAE) [108-01-0] and Selected Salts and Esters: Review of Toxicological Literature (Update) | Report on National Institute of Environmental Health Sciences Contract No. N01-ES-65402 (PDF). National Toxicology Program (Report). Archived (PDF) from the original on 2023-10-04. Retrieved 2015-04-30 – via Contractee Integrated Laboratory Systems, Research Triangle Park, North Carolina, 27709.
- ^ "Sigma Aldrich: Safety Data Sheet: 2-Dimethylaminoethanol (+)-bitartrate". Archived from the original on 2023-10-04.
- ^ Blin, Olivier; Audebert, Christine; Pitel, Séverine; Kaladjian, Arthur; Casse-Perrot, Catherine; Zaim, Mohammed; Micallef, Joelle; Tisne-Versailles, Jacky; Sokoloff, Pierre; Chopin, Philippe; Marien, Marc (2009-12-01). "Effects of dimethylaminoethanol pyroglutamate (DMAE p-Glu) against memory deficits induced by scopolamine: evidence from preclinical and clinical studies". Psychopharmacology. 207 (2): 201–212. doi:10.1007/s00213-009-1648-7. ISSN 1432-2072. PMID 19756528. S2CID 8535134.
- ^ Levin, Edward D; Rose, Jed E; Abood, Leo (June 1995). "Effects of nicotinic dimethylaminoethyl esters on working memory performance of rats in the radial-arm maze". Pharmacology Biochemistry and Behavior. 51 (2–3): 369–373. doi:10.1016/0091-3057(94)00406-9. PMID 7667355. S2CID 20685322.
