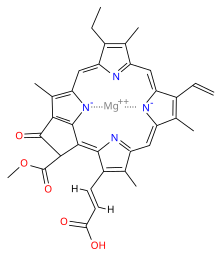
Chlorophyll c
Chlorophyll c refers to forms of chlorophyll found in certain marine algae, including the photosynthetic Chromista (e.g. diatoms and brown algae) and dinoflagellates.[1][2][3] These pigments are characterized by their unusual chemical structure, with a porphyrin as opposed to the chlorin (which has a reduced ring D) as the core; they also do not have an isoprenoid tail. Both these features stand out from the other chlorophylls commonly found in algae and plants.[2]
It has a blue-green color and is an accessory pigment, particularly significant in its absorption of light in the 447–520 nm wavelength region.[3] Like chlorophyll a and chlorophyll b, it helps the organism gather light and passes a quanta of excitation energy through the light harvesting antennae to the photosynthetic reaction centre.[2]
Chlorophyll c can be further divided into chlorophyll c1, chlorophyll c2,[3] and chlorophyll c3,[4] plus at least eight other more recently found subtypes.[5]
Chlorophyll c1
Chlorophyll c1 is a common form of chlorophyll c. It differs from chlorophyll c2 in its C8 group, having an ethyl group instead of vinyl group (C-C single bond instead of C=C double bond). Its absorption maxima are around 444, 577, 626 nm and 447, 579, 629 nm in diethyl ether and acetone respectively.[6]
Chlorophyll c2
Chlorophyll c2 is the most common form of chlorophyll c.[7] Its absorption maxima are around 447, 580, 627 nm and 450, 581, 629 nm in diethyl ether and acetone respectively.[6]
Chlorophyll c3
Chlorophyll c3 is a form of chlorophyll c found in microalga Emiliania huxleyi, identified in 1989.[4] Its absorption maxima are around 452, 585, 625 nm and 452, 585, 627 nm in diethyl ether and acetone respectively.[6]
Biosynthesis
Chlorophyll c synthesis branches off early from the typical Chlorophyllide synthesis pathway, after divinylprotochlorophyllide (DV-PChlide) is formed. It has been established that DV-PChlide and MV-PChlide are processed directly by a 171 oxidase (CHLC, chlorophyll c synthase) into Chl c2 and Chl c1, respectively.[8] The 171 oxidtion was proposed to proceed by "hydroxylation of the 17-propionate reside at the 171-position and successive dehydration to the 17-acrylate residue."[8][9] An 8-vinyl reductase (elaborating on the promiscuous behavior of ferredoxin-type 3,8-divinyl chlorophyllide reductase) could also convert Chl c2 into Chl c1. The two steps could be swapped for the same effect.[10]
Structure
| Chlorophyll c1 | Chlorophyll c2 | Chlorophyll c3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ^ Speer, B. R. "Photosynthetic Pigments". Retrieved 2 August 2014.
- ^ a b c Blankenship RE (February 2002). Molecular Mechanisms of Photosynthesis. Wiley-Blackwell.
- ^ a b c Dougherty RC, Strain HH, Svec WA, Uphaus RA, Katz JJ (May 1970). "The structure, properties, and distribution of chlorophyll c". Journal of the American Chemical Society. 92 (9): 2826–33. Bibcode:1970JAChS..92.2826D. doi:10.1021/ja00712a037. PMID 5439971.
- ^ a b Fookes CJ, Jeffrey SW (1989). "The structure of chlorophyll c3, a novel marine photosynthetic pigment". J. Chem. Soc., Chem. Commun. (23): 1827–28. doi:10.1039/C39890001827.
- ^ Zapata M, Garrido JL, Jeffrey SW (2006). "Chlorophyll c Pigments: Current Status". Chlorophylls and Bacteriochlorophylls: Advances in Photosynthesis and Respiration. Advances in Photosynthesis and Respiration. 25: 39–53. doi:10.1007/1-4020-4516-6_3. ISBN 978-1-4020-4515-8.
- ^ a b c Fawley MW (October 1989). "A new form of chlorophyll C involved in light-harvesting". Plant Physiology. 91 (2): 727–32. doi:10.1104/pp.91.2.727. PMC 1062062. PMID 16667093.
- ^ Jeffrey SW (September 1976). "The Occurrence of Chlorophyll c1 and c2 in Algae". Journal of Phycology. 12 (3): 349–354. Bibcode:1976JPcgy..12..349J. doi:10.1111/j.1529-8817.1976.tb02855.x. S2CID 83927313.
- ^ a b Jiang, Yanyou; Cao, Tianjun; Yang, Yuqing; Zhang, Huan; Zhang, Jingyu; Li, Xiaobo (2023-10-06). "A chlorophyll c synthase widely co-opted by phytoplankton". Science. 382 (6666): 92–98. doi:10.1126/science.adg7921.
- ^ Xu, M; Kinoshita, Y; Matsubara, S; Tamiaki, H (March 2016). "Synthesis of chlorophyll-c derivatives by modifying natural chlorophyll-a". Photosynthesis Research. 127 (3): 335–45. Bibcode:2016PhoRe.127..335X. doi:10.1007/s11120-015-0190-1. PMID 26346903. S2CID 254944200.
- ^ Ito, Hisashi; Tanaka, Ayumi (March 2014). "Evolution of a New Chlorophyll Metabolic Pathway Driven by the Dynamic Changes in Enzyme Promiscuous Activity". Plant and Cell Physiology. 55 (3): 593–603. doi:10.1093/pcp/pct203. hdl:2115/58225. PMID 24399236.