Chloroalanine
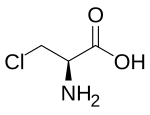 3-Chloro-L-alanine | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H6ClNO2 | |
| Molar mass | 123.54 g·mol−1 |
| Appearance | White solid |
| Melting point | 166–167 °C (331–333 °F; 439–440 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Chloroalanine (3-chloroalanine) is an unnatural amino acid with the formula ClCH2CH(NH2)CO2H. It is a white, water-soluble solid. The compound is usually derived from chlorination of serine. The compound is used in the synthesis of other amino acids by replacement of the chloride.[1] Protected forms of the related iodoalanine are also known.[2][3]
Chemical properties
The hydrolysis of 3-chloro-D-alanine is catalyzed by the enzyme 3-chloro-D-alanine dehydrochlorinase:[4]
- ClCH2CH(NH2)CO2H + H2O →CH3C(O)CO2H + NH4Cl
References
- ^ Hondal, Robert J.; Nilsson, Bradley L.; Raines, Ronald T. (2001). "Selenocysteine in Native Chemical Ligation and Expressed Protein Ligation". Journal of the American Chemical Society. 123 (21): 5140–5141. doi:10.1021/ja005885t. PMID 11457362.
- ^ Richard F. W. Jackson; Manuel Perez-Gonzalez (2005). "Synthesis of N-(Tert-butoxycarbonyl)-β-iodoalanine Methyl Ester: A Useful Building Block in the Synthesis of Nonnatural α-amino Acids via Palladium Catalyzed Cross Coupling Reactions". Org. Synth. 81: 77. doi:10.15227/orgsyn.081.0077.
- ^ Atmuri, N. D. P.; Lubell, W. D. (2015). "Preparation of N-(Boc)-Allylglycine Methyl Ester Using a Zinc-mediated, Palladium-catalyzed Cross-coupling Reaction". Org. Synth. 92: 103. doi:10.15227/orgsyn.092.0103.
- ^ Yamada H, Nagasawa T, Ohkishi H, Kawakami B, Tani Y (June 1981). "Synthesis of D-cysteine from 3-chloro-D-alanine and hydrogen sulfide by 3-chloro-D-alanine hydrogen chloride-lyase (deaminating) of Pseudomonas putida". Biochemical and Biophysical Research Communications. 100 (3): 1104–10. doi:10.1016/0006-291X(81)91937-9. PMID 6791643.
