Atevirdine
| |
| Names | |
|---|---|
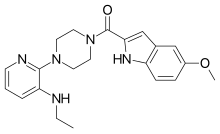
| Preferred IUPAC name {4-[3-(Ethylamino)pyridin-2-yl]piperazin-1-yl}(5-methoxy-1H-indol-2-yl)methanone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H25N5O2 | |
| Molar mass | 379.46 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Atevirdine is a non-nucleoside reverse transcriptase inhibitor that has been studied for the treatment of HIV.[1]
Synthesis

Preparation of the pyridylpiperazine moiety starts by aromatic displacement of chlorine from 2-chloro-3-nitropyridine by piperazine to give 3. The secondary amine is then protected as its BOC derivative by reaction with di-tert-butyl dicarbonate (Boc anhydride) to give 4. The nitro group is then reduced by catalytic hydrogenation. Reductive alkylation with acetaldehyde in the presence of lithium cyanoborohydride gives the corresponding N-ethyl derivative. The protecting group is then removed by reaction with TFA. Reaction of the resulting amine with the imidazolide derivative of 5-methoxy-3-indoleacetic acid produces the amide reverse transcriptase inhibitor, atevirdine.
See also
References
- ^ Morse GD, Reichman RC, Fischl MA, et al. (January 2000). "Concentration-targeted phase I trials of atevirdine mesylate in patients with HIV infection: dosage requirements and pharmacokinetic studies. The ACTG 187 and 199 study teams". Antiviral Res. 45 (1): 47–58. doi:10.1016/S0166-3542(99)00073-X. PMID 10774589.
- ^ D. L. Romero, Drugs Future 19, 9 (1995).
- ^ WO 9109849, Romero, Donna Lee; Mitchell, Mark Allen & Thomas, Richard Charles et al., "Diaromatic substituted anti-AIDS compounds", published 1991-07-11, assigned to Upjohn
- ^ Romero, D. L.; Morge, R. A.; Biles, C.; Berrios-Pena, N.; May, P. D.; Palmer, J. R.; Johnson, P. D.; Smith, H. W.; Busso, M.; et al. (1994). "Discovery, Synthesis, and Bioactivity of Bis(heteroaryl)piperazines. 1. A Novel Class of Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors". Journal of Medicinal Chemistry. 37 (7): 999–1014. doi:10.1021/jm00033a018. PMID 7512142.
