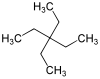
Tetraethylmethane
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 3,3-Diethylpentane[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.151.290 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C9H20 | |||
| Molar mass | 128.259 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Odourless | ||
| Density | 724 mg mL−1 | ||
| Melting point | −34 to −30 °C; −29 to −22 °F; 239 to 243 K | ||
| Boiling point | 145.8 to 146.6 °C; 294.3 to 295.8 °F; 418.9 to 419.7 K | ||
Henry's law constant (kH) |
1.5 nmol Pa−1 kg−1 | ||
| Thermochemistry | |||
Heat capacity (C) |
278.2 J K−1 mol−1 | ||
Std molar entropy (S⦵298) |
333.4 J K−1 mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−6.1261–−6.1229 MJ mol−1 | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related alkanes |
|||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Tetraethylmethane is a branched alkane with 9 carbon atoms. It is a highly flammable and volatile liquid at room temperature. It is one of the isomers of nonane. It is considered one of the most controversial alkanes due to its structural resemblance to the swastika.[2]
References
- ^ "Tetraethylmethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.
- ^ Williams, Matt (2016-09-09). "Uranus & Neptune May Keep "Hitler's Acid" Stable Under Massive Pressure". Universe Today. Retrieved 2024-12-17.
See also
External links
- Alder, Roger W.; Allen, Paul R.; Hnyk, Drahomír; Rankin, David W. H.; Robertson, Heather E.; Smart, Bruce A.; Gillespie, Ronald J.; Bytheway, Ian (1999). "Molecular Structure of 3,3-Diethylpentane (Tetraethylmethane) in the Gas Phase as Determined by Electron Diffraction and ab Initio Calculations". The Journal of Organic Chemistry. 64 (12): 4226–4232. doi:10.1021/jo981779m.



