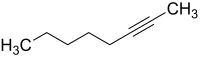
2-Octyne
| |
| Names | |
|---|---|
| Preferred IUPAC name Oct-2-yne | |
| Other names Amylmethylacetylene; Methylpentylacetylene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.018.685 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H14 | |
| Molar mass | 110.200 g·mol−1 |
| Density | 0.759 g/mL |
| Boiling point | 137 °C (279 °F; 410 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
2-Octyne, also known as methylpentylethyne and oct-2-yne,[1] is a type of alkyne with a triple bond at its second carbon (the '2-' indicates the location of the triple bond in the chain). Its formula is C8H14.[2] Its density at 25 °C and otherwise stable conditions is 0.759 g/ml.[3] The boiling point is 137 °C.[3] The average molar mass is 110.20 g/mol.[2]
It is formed by isomerization of 1-octyne catalyzed by a YbII complex.[4]
References
- ^ "2-OCTYNE | C8H14 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 16 August 2016.
- ^ a b Rogers, D. W.; Dagdagan, O. A.; Allinger, N. L. (1979). "webbook.nist.gov/cgi/cbook.cgi". pp. 671–676. Retrieved 16 August 2016.
- ^ a b Sigma-Aldrich Co., 2-Octyne. Retrieved on 16 August 2016.
- ^ Makioka, Yoshikazu; Taniguchi, Yuki; Kitamura, Tsugio; Fujiwara, Yuzo; Saiki, Akira; Takaki, Ken. Isomerization of terminal alkynes catalyzed by ytterbium(II)-aromatic imine complexes. Bulletin de la Société Chimique de France, 1997. Volume 134. (3&4) pp 349-355.
