Metamorphic reaction
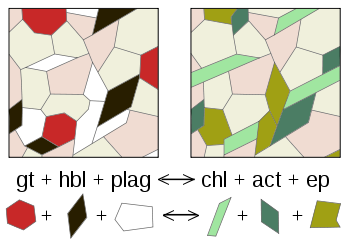
A metamorphic reaction is a chemical reaction that takes place during the geological process of metamorphism wherein one assemblage of minerals is transformed into a second assemblage which is stable under the new temperature/pressure conditions resulting in the final stable state of the observed metamorphic rock.[1]
Examples include the production of talc under varied metamorphic conditions:
- serpentine + carbon dioxide → talc + magnesite + water
- chlorite + quartz → kyanite + talc + water

Polymorphic transformations
Exsolution reactions
Devolatilization reactions
Continuous reactions
Ion exchange reactions
Oxidation/reduction reactions
Reactions involving dissolved species
Chemographics
Petrogenetic grids
Schreinemaker's method
Reaction mechanisms
See also
Notes
- ^ "Types of Metamorphic Reactions". Tulane University. Retrieved 2007-06-22.
