Flubber (material)

Flubber (named from the film The Absent-Minded Professor), Glorp, Glurch, or Slime is a rubbery polymer formed by cross-linking of polyvinyl alcohol (PVA) with a borate compound. Slime can be made by combining polyvinyl-acetate-based adhesives with borax. [1]
Chemical reaction
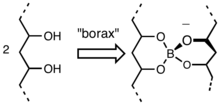
The gelation process entails formation of a borate ester that cross links the chains of the PVA.[2] Borate esters form readily by condensation of hydroxyl groups and the B-OH groups.[3]
Flubber's unique texture comes from a polymer network formed by polyvinyl alcohol. Weak hydrogen bonds hold the polymer chains together, while borate molecules link them side by side. These weak bonds give flubber its distinctive ability to stretch, flow, and pull apart.[4]
Properties
Flubber is a non-Newtonian fluid that flows under low stress, but breaks under higher stresses and pressures. This combination of fluid-like and solid-like properties makes it a Maxwell fluid. Its behavior can also be described as being viscoplastic or gelatinous.[citation needed]
See also
References
- ^ University, Carnegie Mellon. "Polyvinyl Alcohol Slime - Gelfand Center - Carnegie Mellon University". www.cmu.edu. Retrieved 2022-11-01.
- ^ Cassassa, E. Z.; A. M. Sarquis; C. H. Van Dyke (January 1986). "The Gelation of Polyvinyl Alcohol with Borax". Journal of Chemical Education. 63 (1): 57. doi:10.1021/ed063p57. S2CID 94114885.
- ^ Katoa, Y.; K. Suwaa; S. Yokoyamab; T. Yabeb; H. Ikutaa; Y. Uchimotoa; M. Wakihara (December 2002). "Thermally stable solid polymer electrolyte containing borate ester groups for lithium secondary battery". Solid State Ionics. 152–153: 155–159. doi:10.1016/s0167-2738(02)00370-3.
- ^ University, Carnegie Mellon. "Polyvinyl Alcohol Slime - Gelfand Center - Carnegie Mellon University". www.cmu.edu. Retrieved 2022-11-01.
