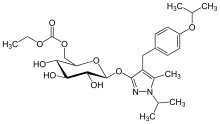
Remogliflozin etabonate
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Remoglifozin is metabolized primarily by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C19 to GSK 279782 (the active metabolite) and GSK 333081 before being glucuronidated to generate inactive glucuronide conjugates.[1] |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H38N2O9 |
| Molar mass | 522.595 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Remogliflozin etabonate (INN/USAN)[2] is a drug of the gliflozin class for the treatment of non-alcoholic steatohepatitis ("NASH") and type 2 diabetes. Remogliflozin was discovered by the Japanese company Kissei Pharmaceutical and is currently being developed by BHV Pharma, a wholly owned subsidiary of North Carolina, US-based Avolynt, and Glenmark Pharmaceuticals through a collaboration with BHV.[3] In 2002, GlaxoSmithKline (GSK) received a license to use it. From 2002 to 2009, GSK carried out a significant clinical development program for the treatment of type-2 diabetes mellitus in various nations across the world and obesity in the UK. Remogliflozin etabonate's pharmacokinetics, pharmacodynamics, and clinical dose regimens were characterized in 18 Phase I and 2 Phase II investigations. Due to financial concerns, GSK stopped working on remogliflozin and sergliflozin, two further SGLT2 inhibitors that were licensed to the company, in 2009.[4] Remogliflozin was commercially launched first in India by Glenmark in May 2019.
Clinical trials
Remogliflozin etabonate was shown to enhance urinary glucose excretion in rodents and humans. Early studies in diabetics improved plasma glucose levels.[5][6] Remogliflozin etabonate has been studied at doses up to 1000 mg.[7] A pair of 12-week phase 2b randomized clinical trials of diabetics published in 2015, found reductions in glycated hemoglobin and that it was generally well tolerated.[8] In a meta-analysis published by Dutta et al. involving data from 3 randomized controlled trials (535 patients), remogliflozin was noted to have similar glycaemic efficacy (reduction in HbA1c and fasting glucose) as compared to dapagliflozin and pioglitazone. [9] A study concluded that concomitant administration of remogliflozin etabonate, either 500 mg or 750 mg BID (twice a day), with metformin 2000 mg BID was safe and effective in patients with type 2 diabetes mellitus during the observation period.[10]
Method of action
Remogliflozin etabonate is a pro-drug of remogliflozin. Remogliflozin inhibits the sodium-glucose transport proteins (SGLT), which are responsible for glucose reabsorption in the kidney. Blocking this transporter causes blood glucose to be eliminated through the urine.[11] Remogliflozin is selective for SGLT2.
See also
References
- ^ Markham, A.J.D., Remogliflozin etabonate: first global approval. 2019. 79(10): p. 1157-1161.
- ^ Statement on a nonproprietory name adopted by the USAN council
- ^ "Avolynt Announces Completion of Phase 2b BRID Study of SGLT2 Inhibitor Remogliflozin-Etabonate" (Press release). Avolynt, Inc. Retrieved July 24, 2018.
- ^ Mohan, V., et al., Remogliflozin etabonate in the treatment of type 2 diabetes: design, development, and place in therapy. 2020: p. 2487-2501.
- ^ Mudaliar S, Armstrong DA, Mavian AA, O'Connor-Semmes R, Mydlow PK, Ye J, et al. (November 2012). "Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes". Diabetes Care. 35 (11): 2198–200. doi:10.2337/dc12-0508. PMC 3476920. PMID 23011728.
- ^ Dobbins RL, O'Connor-Semmes R, Kapur A, Kapitza C, Golor G, Mikoshiba I, et al. (January 2012). "Remogliflozin etabonate, a selective inhibitor of the sodium-dependent transporter 2 reduces serum glucose in type 2 diabetes mellitus patients". Diabetes, Obesity & Metabolism. 14 (1): 15–22. doi:10.1111/j.1463-1326.2011.01462.x. PMID 21733056. S2CID 23372554.
- ^ Sykes AP, O'Connor-Semmes R, Dobbins R, Dorey DJ, Lorimer JD, Walker S, et al. (January 2015). "Randomized trial showing efficacy and safety of twice-daily remogliflozin etabonate for the treatment of type 2 diabetes". Diabetes, Obesity & Metabolism. 17 (1): 94–7. doi:10.1111/dom.12391. PMID 25223369. S2CID 6436562.
- ^ Sykes AP, Kemp GL, Dobbins R, O'Connor-Semmes R, Almond SR, Wilkison WO, et al. (January 2015). "Randomized efficacy and safety trial of once-daily remogliflozin etabonate for the treatment of type 2 diabetes". Diabetes, Obesity & Metabolism. 17 (1): 98–101. doi:10.1111/dom.12393. PMID 25238025. S2CID 25280330.
- ^ Dutta D, Jindal R, Mehta D, Khandelwal D, Sharma M (Nov 2021). "Efficacy and safety of novel sodium glucose cotransporter-2 inhibitor remogliflozin in the management of type 2 diabetes mellitus: A systematic review and meta-analysis". Diabetes Metab Syndr. 15 (6): 102315. doi:10.1016/j.dsx.2021.102315. PMID 34700292. S2CID 239491862.
- ^ Dobbins, R., et al., Assessment of safety and tolerability of remogliflozin etabonate (GSK189075) when administered with total daily dose of 2000 mg of metformin. 2021. 22: p. 1-11.
- ^ "Molecule of the Month: Dapagliflozin". Prous Science. November 2007. Archived from the original on January 6, 2008.
