Pyridoxal
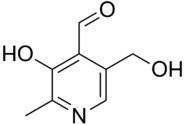 Idealised skeletal formula | |
 Ball-and-stick model based on the crystal structure.[1][2] Note that the acidic phenol group has donated a proton to the basic pyridine group to form a zwitterion, and the hydroxymethyl group has reacted with the aldehyde group to form a hemiacetal. | |
| Names | |
|---|---|
| Preferred IUPAC name 3-Hydroxy-5-(hydroxymethyl)-2-methylpyridine-4-carbaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.573 |
| KEGG | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H9NO3 | |
| Molar mass | 167.16 g/mol |
| Melting point | 165 °C (329 °F; 438 K) (decomposes) |
| Related compounds | |
Related arylformaldehydes |
Damnacanthal |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Pyridoxal (PL)[3] is one form of vitamin B6.
Some medically relevant bacteria, such as those in the genera Granulicatella and Abiotrophia, require pyridoxal for growth. This nutritional requirement can lead to the culture phenomenon of satellite growth. In in vitro culture, these pyridoxal-dependent bacteria may only grow in areas surrounding colonies of bacteria from other genera ("satellitism") that are capable of producing pyridoxal.
Pyridoxal is involved in what is believed to be the most ancient reaction of aerobic metabolism on Earth, about 2.9 billion years ago, a forerunner of the Great Oxidation Event.[4]
See also
References
- ^ "CSD Entry: BIHKEI01". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. 1985. Archived from the original on 2023-11-04. Retrieved 2023-11-04.
- ^ MacLaurin, C. L.; Richardson, M. F. (1985). "Pyridoxal, C8H9NO3, and pyridoxamine dihydrate, C8H12N2O2.2H2O". Acta Crystallogr. C. 41 (2): 261–263. Bibcode:1985AcCrC..41..261M. doi:10.1107/S0108270185003547.
- ^ "Vitamin B-6". iupac.qmul.ac.uk. Retrieved 2024-09-26.
- ^ "Protein Domain Structure Uncovers the Origin of Aerobic Metabolism and the Rise of Planetary Oxygen", Gustavo Caetano-Anolles et al., published in Structure; paper available from University of Illinois News Bureau, 2012.
