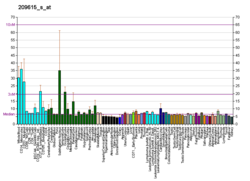
PAK1
Serine/threonine-protein kinase PAK 1 is an enzyme that in humans is encoded by the PAK1 gene.[5][6]
PAK1 is one of six members of the PAK family of serine/threonine kinases which are broadly divided into group I (PAK1, PAK2 and PAK3) and group II (PAK4, PAK6 and PAK5/7).[7][8] The PAKs are evolutionarily conserved.[9] PAK1 localizes in distinct sub-cellular domains in the cytoplasm and nucleus.[10] PAK1 regulates cytoskeleton remodeling, phenotypic signaling and gene expression, and affects a wide variety of cellular processes such as directional motility, invasion, metastasis, growth, cell cycle progression, angiogenesis.[10][11] PAK1-signaling dependent cellular functions regulate both physiologic and disease processes, including cancer, as PAK1 is widely overexpressed and hyperstimulated in human cancer, at-large.[10][12][13]
Discovery
PAK1 was first discovered as an effector of the Rho GTPases in rat brain by Manser and colleagues in 1994.[7] The human PAK1 was identified as a GTP-dependent interacting partner of Rac1 or Cdc42 in the cytosolic fraction from neutrophils, and its complementary DNA was cloned from a human placenta library by Martin and Colleagues in 1995.[8]
Function
PAK proteins are critical effectors that link the Rho family of GTPases (Rho GTPases) to cytoskeleton reorganization and nuclear signaling. PAK proteins, a family of serine/threonine p21-activated kinases, include PAK1, PAK2, PAK3 and PAK4. These proteins serve as targets for the small GTP binding proteins Cdc42 and Rac and have been implicated in a wide range of biological activities. PAK1 regulates cell motility and morphology. Alternative transcripts of this gene have been found, but their full-length natures have not been determined.[14]
Stimulation of PAK1 activity is accompanied by a series of cellular processes that are fundamental to living systems. Being a nodular signaling molecule, PAK1 operates to converging station of a large number of signals triggered by proteins on the cell surface as well as upstream activators, and translates into specific phenotypes. At the biochemical level, these activities are regulated by the ability of PAK1 to phosphorylate its effector interacting substrates, which in-turn set-up a cascade of biochemical events cumulating into a cellular phenotypic response. In addition, PAK1 action is also influenced by its scaffolding activity. Examples of PAK1-regulated cellular processes include dynamic of actin and microtubule fibers, critical steps during cell cycle progression, motility and invasion, redox and energy metabolism, cell survival, angiogenesis, DNA-repair, hormone sensitivity, and gene expression. Functional implications of the PAK1 signaling are exemplified by its role in oncogenesis,[9] viral pathogenesis,[15][16] cardiovascular dysregulation,[17] and neurological disorders.[18]
Gene and spliced variants
The human PAK1 gene is 153-kb long and consists of 23 exons, six exons for 5’-UTR and 17 exons for protein coding (Gene from review). Alternative splicing of six exons generates 20 transcripts from 308-bp to 3.7-kb long; however, only 12 spliced transcripts have open reading frames and are predicted to code ten proteins and two polypeptides. The remaining 8 transcripts range are for non-coding long RNAs from 308-bp to 863-bp long. Unlike the human PAK1, murine PAK1 gene generates five transcripts: three protein-coding from 508-bp to 3.0-kb long, and two transcripts of about 900-bp for non-coding RNAs.
Protein domains
The core domains of the PAK family include a kinase domain in the C-terminal region, a p21-binding domain (PBD), and an auto-inhibitory domain (AID) in group I PAKs. Group I PAKs exist in an inactive, closed homodimer conformation wherein AID of one molecule binds to the kinase domain of another molecule, and activated in both GTPase-dependent and -independent manners.[13]
Activation/inhibition
PAK1 contains an autoinhibitory domain that suppresses the catalytic activity of its kinase domain. PAK1 activators relieve this autoinhibition and initiate conformational rearrangements and autophosphorylation events leading to kinase activation.
IPA-3 (1,1′-disulfanediyldinaphthalen-2-ol) is a small molecule allosteric inhibitor of PAK1. Preactivated PAK1 is resistant to IPA-3. Inhibition in live cells supports a critical role for PAK in PDGF-stimulated ERK activation.[19] Reversible covalent binding of IPA-3 to the PAK1 regulatory domain prevents GTPase docking and the subsequent switch to a catalytically active state.[20]
PAK1 knockdown in prostate cancer cells is associated with reduced motility, reduced MMP9 secretion and increased TGFβ expression, which in these cases, is growth inhibitory. However, IPA-3's pharmacokinetic properties as well as undesirable redox effects in cells, due to the continuous reduction of the sulfhydryl moiety, make it unsuitable for clinical development.[20]
Upstream activators
PAK1 activity is stimulated by a large number of upstream activators and signals, ranging from EGF,[21] heregulin-beta 1,[22] VEGF,[23] basic fibroblast growth factor,[24] platelet-derived growth factor,[25] estrogen,[26] lysophosphatidic acid,[27] phosphoinositides,[28] ETK,[29] AKT,[30] JAK2,[31] ERK,[32] casein kinase II,[33] Rac3,[34] chemokine (C-X-C motif) ligand 1,[35] breast cancer anti-estrogen resistance 3,[36] Kaposi's sarcoma-associated herpesvirus-G protein-coupled receptor,[37] hepatitis B virus X protein,[38] STE20-related kinase adaptor protein α,[39] RhoI,[40] Klotho,[41] N-acetylglucosaminyl transferase V,[42] B-Raf proto-oncogene,[43] casein kinase 2-interacting protein 1,[44] and filamin A.[45]
Downstream effector targets
Functions of PAK1 are regulated by its ability to phosphorylate downstream effector substrates, scaffold activity, redistribution to distinct sub-cellular cellular sub-domains, stimulation or repression of expression of its genomic targets either directly or indirectly, or by all of these mechanisms. Representative PAK1 effector substrates in cancer cells include: Stathmin-S16,[46] Merlin-S518,[47] Vimentin-S25-S38-S50-S65-S72,[48] Histone H3-S10,[49] FilaminA-S2152,[45] Estrogen receptor-alpha-S305,[50] signal transducer and activator of transcription 5a-S779,[51] C-terminal binding protein 1-S158,[52] Raf1-S338,[53] Arpc1b-T21,[54] DLC1-S88,[55] phosphoglucomutase 1-T466,[56] SMART/HDAC1-associated repressor protein-S3486-T3568,[57] Tubulin Cofactor B-S65-S128,[58] Snail-S246 [59] vascular endothelial-cadherin-S665,[60] poly(RC) binding protein 1-T60-S246,[61] integrin-linked kinase 1-T173-S246,[62] epithelium-specific Ets transcription factor 1-S207,[63] ErbB3 binding protein 1-T261,[64] nuclear receptor-interacting factor 3-S28,[65] SRC3-delta4-T56-S659-676,[66] beta-catenin-S675,[67] BAD-S111,[68] BAD-S112, S136,[69] MEK1-S298,[70][71] CRKII-S41,[72] MORC family CW-type zinc finger 2-S739,[73][74] Paxillin-S258,[15] and Paxillin-S273.[75]
Genomic targets
PAK1 and/or PAK1-dependent signals modulate the expression of its genomic targets,[9] including, vascular endothelial growth factor,[23] Cyclin D1,[76] phosphofructokinase-muscle isoform,[77] nuclear factor of activated T-cell,[77] Cyclin B1,[78] Tissue Factor and tissue factor pathway inhibitor,[79] Metalloproteinase 9,[80] and fibronectin.[81]
Interactions
PAK1 has been shown to interact with:
Notes
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000149269 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000030774 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Brown JL, Stowers L, Baer M, Trejo J, Coughlin S, Chant J (May 1996). "Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway". Current Biology. 6 (5): 598–605. Bibcode:1996CBio....6..598B. doi:10.1016/S0960-9822(02)00546-8. PMID 8805275. S2CID 9697114.
- ^ Bekri S, Adélaïde J, Merscher S, Grosgeorge J, Caroli-Bosc F, Perucca-Lostanlen D, Kelley PM, Pébusque MJ, Theillet C, Birnbaum D, Gaudray P (Apr 1998). "Detailed map of a region commonly amplified at 11q13-->q14 in human breast carcinoma". Cytogenetics and Cell Genetics. 79 (1–2): 125–31. doi:10.1159/000134699. PMID 9533029.
- ^ a b Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L (January 1994). "A brain serine/threonine protein kinase activated by Cdc42 and Rac1". Nature. 367 (6458): 40–6. Bibcode:1994Natur.367...40M. doi:10.1038/367040a0. PMID 8107774. S2CID 4332455.
- ^ a b Martin GA, Bollag G, McCormick F, Abo A (May 1995). "A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20". The EMBO Journal. 14 (9): 1970–8. doi:10.1002/j.1460-2075.1995.tb07189.x. PMC 398296. PMID 7744004.
- ^ a b c Kumar A, Molli PR, Pakala SB, Bui Nguyen TM, Rayala SK, Kumar R (July 2009). "PAK thread from amoeba to mammals". Journal of Cellular Biochemistry. 107 (4): 579–85. doi:10.1002/jcb.22159. PMC 2718766. PMID 19350548.
- ^ a b c Vadlamudi RK, Kumar R (December 2003). "P21-activated kinases in human cancer". Cancer and Metastasis Reviews. 22 (4): 385–93. doi:10.1023/a:1023729130497. PMID 12884913. S2CID 5763102.
- ^ Kumar R, Gururaj AE, Barnes CJ (June 2006). "p21-activated kinases in cancer". Nature Reviews. Cancer. 6 (6): 459–71. doi:10.1038/nrc1892. PMID 16723992. S2CID 35272474.
- ^ Radu M, Semenova G, Kosoff R, Chernoff J (January 2014). "PAK signalling during the development and progression of cancer". Nature Reviews. Cancer. 14 (1): 13–25. doi:10.1038/nrc3645. PMC 4115244. PMID 24505617.
- ^ a b Kumar R, Li DQ (2016). PAKs in Human Cancer Progression: From Inception to Cancer Therapeutic to Future Oncobiology. Vol. 130. pp. 137–209. doi:10.1016/bs.acr.2016.01.002. ISBN 978-0-12-804789-7. PMID 27037753.
{{cite book}}:|journal=ignored (help) - ^ "Entrez Gene: PAK1 p21/Cdc42/Rac1-activated kinase 1 (STE20 homolog, yeast)".
- ^ a b Lee JH, Wittki S, Bräu T, Dreyer FS, Krätzel K, Dindorf J, Johnston IC, Gross S, Kremmer E, Zeidler R, Schlötzer-Schrehardt U, Lichtenheld M, Saksela K, Harrer T, Schuler G, Federico M, Baur AS (February 2013). "HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases". Molecular Cell. 49 (4): 668–79. doi:10.1016/j.molcel.2012.12.004. PMID 23317503.
- ^ Van den Broeke C, Radu M, Chernoff J, Favoreel HW (March 2010). "An emerging role for p21-activated kinases (Paks) in viral infections". Trends in Cell Biology. 20 (3): 160–9. doi:10.1016/j.tcb.2009.12.005. PMC 6489496. PMID 20071173.
- ^ Ke Y, Wang X, Jin XY, Solaro RJ, Lei M (December 2014). "PAK1 is a novel cardiac protective signaling molecule". Frontiers of Medicine. 8 (4): 399–403. doi:10.1007/s11684-014-0380-9. PMID 25416031. S2CID 7182791.
- ^ Ma QL, Yang F, Frautschy SA, Cole GM (April 2012). "PAK in Alzheimer disease, Huntington disease and X-linked mental retardation". Cellular Logistics. 2 (2): 117–125. doi:10.4161/cl.21602. PMC 3490962. PMID 23162743.
- ^ Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR (April 2008). "An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase". Chemistry & Biology. 15 (4): 322–31. doi:10.1016/j.chembiol.2008.03.005. PMC 4353635. PMID 18420139.
- ^ a b Goc A, Al-Azayzih A, Abdalla M, Al-Husein B, Kavuri S, Lee J, Moses K, Somanath PR (February 2013). "P21 activated kinase-1 (Pak1) promotes prostate tumor growth and microinvasion via inhibition of transforming growth factor β expression and enhanced matrix metalloproteinase 9 secretion". The Journal of Biological Chemistry. 288 (5): 3025–35. doi:10.1074/jbc.M112.424770. PMC 3561527. PMID 23258534.
- ^ Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J (August 1996). "The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1". The Journal of Biological Chemistry. 271 (35): 20997–1000. doi:10.1074/jbc.271.35.20997. PMID 8798379.
- ^ Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R (October 1998). "Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase". The Journal of Biological Chemistry. 273 (43): 28238–46. doi:10.1074/jbc.273.43.28238. PMID 9774445.
- ^ a b Bagheri-Yarmand R, Vadlamudi RK, Wang RA, Mendelsohn J, Kumar R (December 2000). "Vascular endothelial growth factor up-regulation via p21-activated kinase-1 signaling regulates heregulin-beta1-mediated angiogenesis". The Journal of Biological Chemistry. 275 (50): 39451–7. doi:10.1074/jbc.M006150200. PMID 10967114.
- ^ Shin KS, Shin EY, Lee CS, Quan SH, Woo KN, Soung NK, Kwak SJ, Kim SR, Kim EG (May 2002). "Basic fibroblast growth factor-induced translocation of p21-activated kinase to the membrane is independent of phospholipase C-gamma1 in the differentiation of PC12 cells". Experimental & Molecular Medicine. 34 (2): 172–6. doi:10.1038/emm.2002.25. PMID 12085993.
- ^ He H, Levitzki A, Zhu HJ, Walker F, Burgess A, Maruta H (July 2001). "Platelet-derived growth factor requires epidermal growth factor receptor to activate p21-activated kinase family kinases". The Journal of Biological Chemistry. 276 (29): 26741–4. doi:10.1074/jbc.C100229200. PMID 11356824.
- ^ Mazumdar A, Kumar R (January 2003). "Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells". FEBS Letters. 535 (1–3): 6–10. Bibcode:2003FEBSL.535....6M. doi:10.1016/s0014-5793(02)03846-2. PMID 12560069. S2CID 28855687.
- ^ Jung ID, Lee J, Lee KB, Park CG, Kim YK, Seo DW, Park D, Lee HW, Han JW, Lee HY (April 2004). "Activation of p21-activated kinase 1 is required for lysophosphatidic acid-induced focal adhesion kinase phosphorylation and cell motility in human melanoma A2058 cells". European Journal of Biochemistry. 271 (8): 1557–65. doi:10.1111/j.1432-1033.2004.04066.x. PMID 15066181.
- ^ Strochlic TI, Viaud J, Rennefahrt UE, Anastassiadis T, Peterson JR (November 2010). "Phosphoinositides are essential coactivators for p21-activated kinase 1". Molecular Cell. 40 (3): 493–500. doi:10.1016/j.molcel.2010.10.015. PMC 3026281. PMID 21070974.
- ^ a b Bagheri-Yarmand R, Mandal M, Taludker AH, Wang RA, Vadlamudi RK, Kung HJ, Kumar R (August 2001). "Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells". The Journal of Biological Chemistry. 276 (31): 29403–9. doi:10.1074/jbc.M103129200. PMID 11382770.
- ^ Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J (November 2003). "Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration". Molecular and Cellular Biology. 23 (22): 8058–69. doi:10.1128/mcb.23.22.8058-8069.2003. PMC 262366. PMID 14585966.
- ^ Rider L, Shatrova A, Feener EP, Webb L, Diakonova M (October 2007). "JAK2 tyrosine kinase phosphorylates PAK1 and regulates PAK1 activity and functions". The Journal of Biological Chemistry. 282 (42): 30985–96. doi:10.1074/jbc.M701794200. PMID 17726028.
- ^ Yuan L, Santi M, Rushing EJ, Cornelison R, MacDonald TJ (October 2010). "ERK activation of p21 activated kinase-1 (Pak1) is critical for medulloblastoma cell migration". Clinical & Experimental Metastasis. 27 (7): 481–91. doi:10.1007/s10585-010-9337-9. PMC 2954413. PMID 20526801.
- ^ Shin YJ, Kim YB, Kim JH (September 2013). "Protein kinase CK2 phosphorylates and activates p21-activated kinase 1". Molecular Biology of the Cell. 24 (18): 2990–9. doi:10.1091/mbc.E13-04-0204. PMC 3771959. PMID 23885116.
- ^ Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG (January 2000). "Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway". Proceedings of the National Academy of Sciences of the United States of America. 97 (1): 185–9. Bibcode:2000PNAS...97..185M. doi:10.1073/pnas.97.1.185. PMC 26637. PMID 10618392.
- ^ Wang D, Sai J, Richmond A (February 2003). "Cell surface heparan sulfate participates in CXCL1-induced signaling". Biochemistry. 42 (4): 1071–7. doi:10.1021/bi026425a. PMC 2667446. PMID 12549928.
- ^ Cai D, Iyer A, Felekkis KN, Near RI, Luo Z, Chernoff J, Albanese C, Pestell RG, Lerner A (October 2003). "AND-34/BCAR3, a GDP exchange factor whose overexpression confers antiestrogen resistance, activates Rac, PAK1, and the cyclin D1 promoter". Cancer Research. 63 (20): 6802–8. PMID 14583477.
- ^ Dadke D, Fryer BH, Golemis EA, Field J (December 2003). "Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi's sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation". Cancer Research. 63 (24): 8837–47. PMID 14695200.
- ^ Xu J, Liu H, Chen L, Wang S, Zhou L, Yun X, Sun L, Wen Y, Gu J (July 2012). "Hepatitis B virus X protein confers resistance of hepatoma cells to anoikis by up-regulating and activating p21-activated kinase 1". Gastroenterology. 143 (1): 199–212.e4. doi:10.1053/j.gastro.2012.03.053. PMID 22484303.
- ^ Eggers CM, Kline ER, Zhong D, Zhou W, Marcus AI (May 2012). "STE20-related kinase adaptor protein α (STRADα) regulates cell polarity and invasion through PAK1 signaling in LKB1-null cells". The Journal of Biological Chemistry. 287 (22): 18758–68. doi:10.1074/jbc.M111.316422. PMC 3365778. PMID 22493453.
- ^ Ho H, Aruri J, Kapadia R, Mehr H, White MA, Ganesan AK (November 2012). "RhoJ regulates melanoma chemoresistance by suppressing pathways that sense DNA damage". Cancer Research. 72 (21): 5516–28. doi:10.1158/0008-5472.CAN-12-0775. PMC 3548429. PMID 22971344.
- ^ Chen L, Liu H, Liu J, Zhu Y, Xu L, He H, Zhang H, Wang S, Wu Q, Liu W, Liu Y, Pan D, Ren S, Xu J, Gu J (2013). "Klotho endows hepatoma cells with resistance to anoikis via VEGFR2/PAK1 activation in hepatocellular carcinoma". PLOS ONE. 8 (3): e58413. Bibcode:2013PLoSO...858413C. doi:10.1371/journal.pone.0058413. PMC 3596390. PMID 23516476.
- ^ Liu J, Liu H, Zhang W, Wu Q, Liu W, Liu Y, Pan D, Xu J, Gu J (September 2013). "N-acetylglucosaminyltransferase V confers hepatoma cells with resistance to anoikis through EGFR/PAK1 activation". Glycobiology. 23 (9): 1097–109. doi:10.1093/glycob/cwt049. PMID 23811795.
- ^ McCarty SK, Saji M, Zhang X, Knippler CM, Kirschner LS, Fernandez S, Ringel MD (2014). "BRAF activates and physically interacts with PAK to regulate cell motility". Endocrine-Related Cancer. 21 (6): 865–77. doi:10.1530/ERC-14-0424. PMC 4487662. PMID 25228413.
- ^ Kim YB, Shin YJ, Roy A, Kim JH (August 2015). "The Role of the Pleckstrin Homology Domain-containing Protein CKIP-1 in Activation of p21-activated Kinase 1 (PAK1)". The Journal of Biological Chemistry. 290 (34): 21076–85. doi:10.1074/jbc.M115.675124. PMC 4543665. PMID 26160174.
- ^ a b Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R (September 2002). "Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1". Nature Cell Biology. 4 (9): 681–90. doi:10.1038/ncb838. PMID 12198493. S2CID 36460759.
- ^ Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A (January 2001). "Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16". The Journal of Biological Chemistry. 276 (3): 1677–80. doi:10.1074/jbc.C000635200. PMID 11058583.
- ^ Xiao GH, Beeser A, Chernoff J, Testa JR (January 2002). "p21-activated kinase links Rac/Cdc42 signaling to merlin". The Journal of Biological Chemistry. 277 (2): 883–6. doi:10.1074/jbc.C100553200. PMID 11719502.
- ^ Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M (February 2002). "Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK)". Genes to Cells. 7 (2): 91–7. doi:10.1046/j.1356-9597.2001.00504.x. PMID 11895474. S2CID 28180002.
- ^ Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, Mandal M, Kumar R (August 2002). "p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells". EMBO Reports. 3 (8): 767–73. doi:10.1093/embo-reports/kvf157. PMC 1084211. PMID 12151336.
- ^ Wang RA, Mazumdar A, Vadlamudi RK, Kumar R (October 2002). "P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium". The EMBO Journal. 21 (20): 5437–47. doi:10.1093/emboj/cdf543. PMC 129075. PMID 12374744.
- ^ Wang RA, Vadlamudi RK, Bagheri-Yarmand R, Beuvink I, Hynes NE, Kumar R (May 2003). "Essential functions of p21-activated kinase 1 in morphogenesis and differentiation of mammary glands". The Journal of Cell Biology. 161 (3): 583–92. doi:10.1083/jcb.200212066. PMC 2172951. PMID 12732616.
- ^ Barnes CJ, Vadlamudi RK, Mishra SK, Jacobson RH, Li F, Kumar R (August 2003). "Functional inactivation of a transcriptional corepressor by a signaling kinase". Nature Structural Biology. 10 (8): 622–8. doi:10.1038/nsb957. PMID 12872159. S2CID 12312851.
- ^ Gurdon JB (January 1992). "The generation of diversity and pattern in animal development". Cell. 68 (2): 185–99. doi:10.1016/0092-8674(92)90465-o. PMID 1733498. S2CID 43600561.
- ^ Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R (February 2004). "p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate". EMBO Reports. 5 (2): 154–60. doi:10.1038/sj.embor.7400079. PMC 1298990. PMID 14749719.
- ^ a b Vadlamudi RK, Bagheri-Yarmand R, Yang Z, Balasenthil S, Nguyen D, Sahin AA, den Hollander P, Kumar R (June 2004). "Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes". Cancer Cell. 5 (6): 575–85. doi:10.1016/j.ccr.2004.05.022. PMID 15193260.
- ^ Gururaj A, Barnes CJ, Vadlamudi RK, Kumar R (October 2004). "Regulation of phosphoglucomutase 1 phosphorylation and activity by a signaling kinase". Oncogene. 23 (49): 8118–27. doi:10.1038/sj.onc.1207969. PMID 15378030.
- ^ Vadlamudi RK, Manavathi B, Singh RR, Nguyen D, Li F, Kumar R (June 2005). "An essential role of Pak1 phosphorylation of SHARP in Notch signaling". Oncogene. 24 (28): 4591–6. doi:10.1038/sj.onc.1208672. PMID 15824732.
- ^ Vadlamudi RK, Barnes CJ, Rayala S, Li F, Balasenthil S, Marcus S, Goodson HV, Sahin AA, Kumar R (May 2005). "p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B". Molecular and Cellular Biology. 25 (9): 3726–36. doi:10.1128/MCB.25.9.3726-3736.2005. PMC 1084301. PMID 15831477.
- ^ Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R (April 2005). "Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions". Cancer Research. 65 (8): 3179–84. doi:10.1158/0008-5472.CAN-04-3480. PMID 15833848.
- ^ Gavard J, Gutkind JS (November 2006). "VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin". Nature Cell Biology. 8 (11): 1223–34. doi:10.1038/ncb1486. PMID 17060906. S2CID 36686511.
- ^ Meng Q, Rayala SK, Gururaj AE, Talukder AH, O'Malley BW, Kumar R (April 2007). "Signaling-dependent and coordinated regulation of transcription, splicing, and translation resides in a single coregulator, PCBP1". Proceedings of the National Academy of Sciences of the United States of America. 104 (14): 5866–71. Bibcode:2007PNAS..104.5866M. doi:10.1073/pnas.0701065104. PMC 1851583. PMID 17389360.
- ^ Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R (April 2007). "Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase". Proceedings of the National Academy of Sciences of the United States of America. 104 (16): 6782–7. Bibcode:2007PNAS..104.6782A. doi:10.1073/pnas.0701999104. PMC 1871862. PMID 17420447.
- ^ Manavathi B, Rayala SK, Kumar R (July 2007). "Phosphorylation-dependent regulation of stability and transforming potential of ETS transcriptional factor ESE-1 by p21-activated kinase 1". The Journal of Biological Chemistry. 282 (27): 19820–30. doi:10.1074/jbc.M702309200. PMID 17491012.
- ^ Akinmade D, Talukder AH, Zhang Y, Luo WM, Kumar R, Hamburger AW (March 2008). "Phosphorylation of the ErbB3 binding protein Ebp1 by p21-activated kinase 1 in breast cancer cells". British Journal of Cancer. 98 (6): 1132–40. doi:10.1038/sj.bjc.6604261. PMC 2275482. PMID 18283314.
- ^ Talukder AH, Li DQ, Manavathi B, Kumar R (September 2008). "Serine 28 phosphorylation of NRIF3 confers its co-activator function for estrogen receptor-alpha transactivation". Oncogene. 27 (39): 5233–42. doi:10.1038/onc.2008.151. PMC 3621709. PMID 18521086.
- ^ Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R, Mancini MA, Tsai SY, Tsai MJ, O'Malley BW (February 2010). "SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration". Molecular Cell. 37 (3): 321–32. doi:10.1016/j.molcel.2010.01.004. PMC 2824333. PMID 20159552.
- ^ Zhu G, Wang Y, Huang B, Liang J, Ding Y, Xu A, Wu W (February 2012). "A Rac1/PAK1 cascade controls β-catenin activation in colon cancer cells". Oncogene. 31 (8): 1001–12. doi:10.1038/onc.2011.294. PMID 21822311.
- ^ Ye DZ, Jin S, Zhuo Y, Field J (2011). "p21-Activated kinase 1 (Pak1) phosphorylates BAD directly at serine 111 in vitro and indirectly through Raf-1 at serine 112". PLOS ONE. 6 (11): e27637. Bibcode:2011PLoSO...627637Y. doi:10.1371/journal.pone.0027637. PMC 3214075. PMID 22096607.
- ^ Schürmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM (January 2000). "p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis". Molecular and Cellular Biology. 20 (2): 453–61. doi:10.1128/mcb.20.2.453-461.2000. PMC 85099. PMID 10611223.
- ^ Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J, Wang S, Barretina J, Weir BA, Zhao JJ, Polyak K, Golub TR, Beroukhim R, Hahn WC (July 2012). "PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling" (PDF). Oncogene. 31 (29): 3397–408. doi:10.1038/onc.2011.515. PMC 3291810. PMID 22105362.
- ^ Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, Catling AD (July 2003). "PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation". The Journal of Cell Biology. 162 (2): 281–91. doi:10.1083/jcb.200212141. PMC 2172784. PMID 12876277.
- ^ Rettig M, Trinidad K, Pezeshkpour G, Frost P, Sharma S, Moatamed F, Tamanoi F, Mortazavi F (2012). "PAK1 kinase promotes cell motility and invasiveness through CRK-II serine phosphorylation in non-small cell lung cancer cells". PLOS ONE. 7 (7): e42012. Bibcode:2012PLoSO...742012R. doi:10.1371/journal.pone.0042012. PMC 3407072. PMID 22848689.
- ^ Li DQ, Nair SS, Ohshiro K, Kumar A, Nair VS, Pakala SB, Reddy SD, Gajula RP, Eswaran J, Aravind L, Kumar R (December 2012). "MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response". Cell Reports. 2 (6): 1657–69. doi:10.1016/j.celrep.2012.11.018. PMC 3554793. PMID 23260667.
- ^ Wang G, Song Y, Liu T, Wang C, Zhang Q, Liu F, Cai X, Miao Z, Xu H, Xu H, Cao L, Li F (2015). "PAK1-mediated MORC2 phosphorylation promotes gastric tumorigenesis". Oncotarget. 6 (12): 9877–86. doi:10.18632/oncotarget.3185. PMC 4496403. PMID 25888627.
- ^ Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR (May 2006). "Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics". The Journal of Cell Biology. 173 (4): 587–9. doi:10.1083/jcb.200509075. PMC 2063867. PMID 16717130.
- ^ Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R (January 2004). "p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells". The Journal of Biological Chemistry. 279 (2): 1422–8. doi:10.1074/jbc.M309937200. PMID 14530270.
- ^ a b Singh RR, Song C, Yang Z, Kumar R (May 2005). "Nuclear localization and chromatin targets of p21-activated kinase 1". The Journal of Biological Chemistry. 280 (18): 18130–7. doi:10.1074/jbc.M412607200. PMID 15749698.
- ^ Liu F, Li X, Wang C, Cai X, Du Z, Xu H, Li F (December 2009). "Downregulation of p21-activated kinase-1 inhibits the growth of gastric cancer cells involving cyclin B1". International Journal of Cancer. 125 (11): 2511–9. doi:10.1002/ijc.24588. PMID 19610058. S2CID 43415843.
- ^ Sánchez-Solana B, Motwani M, Li DQ, Eswaran J, Kumar R (November 2012). "p21-activated kinase-1 signaling regulates transcription of tissue factor and tissue factor pathway inhibitor". The Journal of Biological Chemistry. 287 (47): 39291–302. doi:10.1074/jbc.M112.404061. PMC 3501013. PMID 23038262.
- ^ Goc A, Al-Azayzih A, Abdalla M, Al-Husein B, Kavuri S, Lee J, Moses K, Somanath PR (February 2013). "P21 activated kinase-1 (Pak1) promotes prostate tumor growth and microinvasion via inhibition of transforming growth factor β expression and enhanced matrix metalloproteinase 9 secretion". The Journal of Biological Chemistry. 288 (5): 3025–35. doi:10.1074/jbc.M112.424770. PMC 3561527. PMID 23258534.
- ^ Jagadeeshan S, Krishnamoorthy YR, Singhal M, Subramanian A, Mavuluri J, Lakshmi A, Roshini A, Baskar G, Ravi M, Joseph LD, Sadasivan K, Krishnan A, Nair AS, Venkatraman G, Rayala SK (January 2015). "Transcriptional regulation of fibronectin by p21-activated kinase-1 modulates pancreatic tumorigenesis". Oncogene. 34 (4): 455–64. doi:10.1038/onc.2013.576. PMID 24561527. S2CID 23631950.
- ^ Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM (April 2004). "p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor". The Journal of Biological Chemistry. 279 (18): 18392–400. doi:10.1074/jbc.M400084200. PMID 14970201.
- ^ Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R (February 2004). "p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate". EMBO Reports. 5 (2): 154–60. doi:10.1038/sj.embor.7400079. PMC 1298990. PMID 14749719.
- ^ Zang M, Hayne C, Luo Z (February 2002). "Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1". The Journal of Biological Chemistry. 277 (6): 4395–405. doi:10.1074/jbc.M110000200. PMID 11733498.
- ^ a b Seoh ML, Ng CH, Yong J, Lim L, Leung T (March 2003). "ArhGAP15, a novel human RacGAP protein with GTPase binding property". FEBS Letters. 539 (1–3): 131–7. doi:10.1016/s0014-5793(03)00213-8. PMID 12650940. S2CID 27574424.
- ^ a b Zhang B, Chernoff J, Zheng Y (April 1998). "Interaction of Rac1 with GTPase-activating proteins and putative effectors. A comparison with Cdc42 and RhoA". The Journal of Biological Chemistry. 273 (15): 8776–82. doi:10.1074/jbc.273.15.8776. PMID 9535855.
- ^ Rashid T, Banerjee M, Nikolic M (December 2001). "Phosphorylation of Pak1 by the p35/Cdk5 kinase affects neuronal morphology". The Journal of Biological Chemistry. 276 (52): 49043–52. doi:10.1074/jbc.M105599200. PMID 11604394.
- ^ Edwards DC, Sanders LC, Bokoch GM, Gill GN (September 1999). "Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics". Nature Cell Biology. 1 (5): 253–9. doi:10.1038/12963. PMID 10559936. S2CID 25250183.
- ^ Ku GM, Yablonski D, Manser E, Lim L, Weiss A (February 2001). "A PAK1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT". The EMBO Journal. 20 (3): 457–65. doi:10.1093/emboj/20.3.457. PMC 133476. PMID 11157752.
- ^ Braverman LE, Quilliam LA (February 1999). "Identification of Grb4/Nckbeta, a src homology 2 and 3 domain-containing adapter protein having similar binding and biological properties to Nck". The Journal of Biological Chemistry. 274 (9): 5542–9. doi:10.1074/jbc.274.9.5542. PMID 10026169.
- ^ Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG (October 1996). "Interaction of the Nck adapter protein with p21-activated kinase (PAK1)". The Journal of Biological Chemistry. 271 (42): 25746–9. doi:10.1074/jbc.271.42.25746. PMID 8824201.
- ^ Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M (May 2001). "Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1". Proceedings of the National Academy of Sciences of the United States of America. 98 (11): 6174–9. Bibcode:2001PNAS...98.6174X. doi:10.1073/pnas.101137298. PMC 33441. PMID 11371639.
- ^ Katoh H, Negishi M (July 2003). "RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo". Nature. 424 (6947): 461–4. Bibcode:2003Natur.424..461K. doi:10.1038/nature01817. PMID 12879077. S2CID 4411133.
External links
- PAK1 Info with links in the Cell Migration Gateway Archived 2014-12-11 at the Wayback Machine
- Andrei M (March 23, 2016). "Researchers zoom in on potential treatment for prostate cancer". ZME Science. Retrieved 2016-04-23.