SCN8A
| SCN8A | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SCN8A, CERIII, CIAT, EIEE13, MED, NaCh6, Nav1.6, PN4, sodium voltage-gated channel alpha subunit 8, BFIS5, MYOCL2, DEE13 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 600702; MGI: 103169; HomoloGene: 7927; GeneCards: SCN8A; OMA:SCN8A - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Sodium channel protein type 8 subunit alpha also known as Nav1.6 is a membrane protein encoded by the SCN8A gene.[5][6] Nav1.6 is one sodium channel isoform and is the primary voltage-gated sodium channel at each node of Ranvier. The channels are highly concentrated in sensory and motor axons in the peripheral nervous system and cluster at the nodes in the central nervous system.[7][8][9]
Structure
Nav1.6 is encoded by the SCN8A gene which contains 27 exons and measures 170 kb. The voltage gated sodium channel is composed of 1980 residues. Like other sodium channels, Nav1.6 is a monomer composed of four homologous domains (I-IV) and 25 transmembrane segments. SCN8A encodes S3-S4 transmembrane segments which form an intracellular loop.[10]
Function
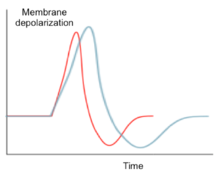
Like other sodium ion channels, Nav1.6 facilitates action potential propagation when the membrane potential is depolarized by an influx of Na+ ions. However, Nav1.6 is able to sustain repetitive excitation and firing. The high frequency firing characteristic of Nav1.6 is caused by a persistent and resurgent sodium current. This characteristic is caused by slow activation of the sodium channel following repolarization,[11] which allows a steady-state sodium current after the initial action potential propagation. The steady-state sodium current contributes to the depolarization of the following action potential. Additionally, the activation threshold of Nav1.6 is lower compared to other common sodium channels such as Nav1.2. This feature allows Nav1.6 channels to rapidly recover from inactivation and sustain a high rate of activity.[12]
Nav1.6 is expressed primarily in the nodes of Ranvier in myelinated axons but is also highly concentrated at the distal end of the axon hillock, cerebellar granule cells and Purkinje neurons and to a lower extent in non-myelinated axons and dendrites.[12] Given the location of Nav1.6, the channel contributes to the firing threshold of a given neuron, as the electrical impulses from various inputs are summed at the axon hillock in order to reach firing threshold before propagating down the axon. Other sodium channel isoforms are expressed at the distal end of the axon hillock, including Nav1.1 and Nav1.2.[8]

NaV1.6 channels demonstrate resistance against protein phosphorylation regulation. Sodium channels are modulated by protein kinase A and protein kinase C (PKC) phosphorylation, which reduce peak sodium currents. Dopamine and acetylcholine decrease sodium currents in hippocampal pyramidal neurons through phosphorylation. Similarly, serotonin receptors in the prefrontal cortex are regulated by PKC in order to reduce sodium currents.[11] Phosphorylated regulation in sodium channels helps to slow inactivation. However, NaV1.6 channels lacks adequate protein kinase sites. Phosphorylation sites at amino acid residues Ser573 and Ser687 are found in other sodium channels but are not well conserved in NaV1.6. The lack of serine residues lead to the channel's ability to consistently and quickly fire following inactivation.[14]
NaV1.6 is conversely regulated by Calmodulin (CaM). CaM interacts with the isoleucine-glutamine (IQ) motif of NaV1.6 in order to inactivate the channel. The IQ motif folds into a helix when interacting with CaM and CaM will inactivate NaV1.6 depending on the concentration of calcium. The NaV1.6 IQ demonstrates moderate affinity for CaM compared to other sodium channel isoforms such as NaV1.6. The difference in CaM affinity contributes to NaV1.6's resistance to inactivation.[15]
Clinical significance
The first known mutation in humans was discovered by Krishna Veeramah and Michael Hammer in 2012.[16] The genome of a child demonstrating epileptic encephalopathy was sequenced and revealed a de novo missense mutation, p.Asn1768Asp. The missense mutations in Nav1.6 increased channel function by increasing the duration of the persistent sodium current and prevented complete inactivation following hyperpolarization. 20% of the initial current persisted 100 ms after hyperpolarization resulting in hyperexcitability of the neuron and increasing the likelihood of premature or unintentional firing. In addition to epileptic encephalopathy, the patient presented with developmental delay, autistic features, intellectual disability and ataxia.
Sodium channel conversion has been implicated in the demyelination of axons related multiple sclerosis (MS). In early stages of myelination, immature Nav1.2 channels outnumber Nav1.6 in axons. However, mature Nav1.6 channels gradually replace the other channels as myelination continues, allowing increased conduction velocity given the lower threshold of Nav1.6.[8] However, in MS models, sodium channel conversion from mature Nav1.6 to Nav1.2 is observed.[17]
See also
- Sodium channel
- paralytic - SCN8A ortholog in Drosophila
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000196876 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000023033 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "UniProt". www.uniprot.org. Retrieved 25 July 2022.
- ^ "Entrez Gene: SCN8A sodium channel, voltage gated, type VIII, alpha subunit".
- ^ Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR (May 2000). "Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses". Proceedings of the National Academy of Sciences of the United States of America. 97 (10): 5616–20. Bibcode:2000PNAS...97.5616C. doi:10.1073/pnas.090034797. PMC 25877. PMID 10779552.
- ^ a b c Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G (April 2001). "Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon". Neuron. 30 (1): 91–104. doi:10.1016/s0896-6273(01)00265-3. PMID 11343647. S2CID 7168889.
- ^ Tzoumaka E, Tischler AC, Sangameswaran L, Eglen RM, Hunter JC, Novakovic SD (April 2000). "Differential distribution of the tetrodotoxin-sensitive rPN4/NaCh6/Scn8a sodium channel in the nervous system". Journal of Neuroscience Research. 60 (1): 37–44. doi:10.1002/(SICI)1097-4547(20000401)60:1<37::AID-JNR4>3.0.CO;2-W. PMID 10723066. S2CID 46539625.
- ^ O'Brien JE, Meisler MH (October 2013). "Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability". Frontiers in Genetics. 4: 213. doi:10.3389/fgene.2013.00213. PMC 3809569. PMID 24194747.
- ^ a b Chen Y, Yu FH, Sharp EM, Beacham D, Scheuer T, Catterall WA (August 2008). "Functional properties and differential neuromodulation of Na(v)1.6 channels". Molecular and Cellular Neurosciences. 38 (4): 607–15. doi:10.1016/j.mcn.2008.05.009. PMC 3433175. PMID 18599309.
- ^ a b Freeman SA, Desmazières A, Fricker D, Lubetzki C, Sol-Foulon N (February 2016). "Mechanisms of sodium channel clustering and its influence on axonal impulse conduction". Cellular and Molecular Life Sciences. 73 (4): 723–35. doi:10.1007/s00018-015-2081-1. PMC 4735253. PMID 26514731.
- ^ Reddy Chichili VP, Xiao Y, Seetharaman J, Cummins TR, Sivaraman J (2013). "Structural basis for the modulation of the neuronal voltage-gated sodium channel NaV1.6 by calmodulin". Scientific Reports. 3: 2435. Bibcode:2013NatSR...3E2435C. doi:10.1038/srep02435. PMC 3743062. PMID 23942337.
- ^ Chen Y, Yu FH, Sharp EM, Beacham D, Scheuer T, Catterall WA (August 2008). "Functional properties and differential neuromodulation of Na(v)1.6 channels". Molecular and Cellular Neurosciences. 38 (4): 607–15. doi:10.1016/j.mcn.2008.05.009. PMC 3433175. PMID 18599309.
- ^ Reddy Chichili VP, Xiao Y, Seetharaman J, Cummins TR, Sivaraman J (2013-08-14). "Structural basis for the modulation of the neuronal voltage-gated sodium channel NaV1.6 by calmodulin". Scientific Reports. 3: 2435. Bibcode:2013NatSR...3E2435C. doi:10.1038/srep02435. PMC 3743062. PMID 23942337.
- ^ Veeramah KR, O'Brien JE, Meisler MH, Cheng X, Dib-Hajj SD, Waxman SG, Talwar D, Girirajan S, Eichler EE, Restifo LL, Erickson RP, Hammer MF (March 2012). "De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP". American Journal of Human Genetics. 90 (3): 502–10. doi:10.1016/j.ajhg.2012.01.006. PMC 3309181. PMID 22365152.
- ^ Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG (May 2004). "Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger". Proceedings of the National Academy of Sciences of the United States of America. 101 (21): 8168–73. Bibcode:2004PNAS..101.8168C. doi:10.1073/pnas.0402765101. PMC 419575. PMID 15148385.
Further reading
- Catterall WA, Goldin AL, Waxman SG (December 2005). "International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels". Pharmacological Reviews. 57 (4): 397–409. doi:10.1124/pr.57.4.4. PMID 16382098. S2CID 7332624.
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH (August 1995). "Mutation of a new sodium channel gene, Scn8a, in the mouse mutant 'motor endplate disease'". Nature Genetics. 10 (4): 461–5. doi:10.1038/ng0895-461. PMID 7670495. S2CID 28941670.
- Plummer NW, McBurney MW, Meisler MH (September 1997). "Alternative splicing of the sodium channel SCN8A predicts a truncated two-domain protein in fetal brain and non-neuronal cells". The Journal of Biological Chemistry. 272 (38): 24008–15. doi:10.1074/jbc.272.38.24008. PMID 9295353.
- Plummer NW, Galt J, Jones JM, Burgess DL, Sprunger LK, Kohrman DC, Meisler MH (December 1998). "Exon organization, coding sequence, physical mapping, and polymorphic intragenic markers for the human neuronal sodium channel gene SCN8A". Genomics. 54 (2): 287–96. doi:10.1006/geno.1998.5550. PMID 9828131.
- Anis Y, Nürnberg B, Visochek L, Reiss N, Naor Z, Cohen-Armon M (March 1999). "Activation of Go-proteins by membrane depolarization traced by in situ photoaffinity labeling of galphao-proteins with [alpha32P]GTP-azidoanilide". The Journal of Biological Chemistry. 274 (11): 7431–40. doi:10.1074/jbc.274.11.7431. PMID 10066808.
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR (May 2000). "Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses". Proceedings of the National Academy of Sciences of the United States of America. 97 (10): 5616–20. Bibcode:2000PNAS...97.5616C. doi:10.1073/pnas.090034797. PMC 25877. PMID 10779552.
- Wittmack EK, Rush AM, Craner MJ, Goldfarb M, Waxman SG, Dib-Hajj SD (July 2004). "Fibroblast growth factor homologous factor 2B: association with Nav1.6 and selective colocalization at nodes of Ranvier of dorsal root axons". The Journal of Neuroscience. 24 (30): 6765–75. doi:10.1523/JNEUROSCI.1628-04.2004. PMC 6729706. PMID 15282281.
- Raymond CK, Castle J, Garrett-Engele P, Armour CD, Kan Z, Tsinoremas N, Johnson JM (October 2004). "Expression of alternatively spliced sodium channel alpha-subunit genes. Unique splicing patterns are observed in dorsal root ganglia". The Journal of Biological Chemistry. 279 (44): 46234–41. doi:10.1074/jbc.M406387200. PMID 15302875.
- Drews VL, Lieberman AP, Meisler MH (February 2005). "Multiple transcripts of sodium channel SCN8A (Na(V)1.6) with alternative 5'- and 3'-untranslated regions and initial characterization of the SCN8A promoter". Genomics. 85 (2): 245–57. doi:10.1016/j.ygeno.2004.09.002. PMID 15676283.
- Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD (July 2005). "Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase". The Journal of Neuroscience. 25 (28): 6621–30. doi:10.1523/JNEUROSCI.0541-05.2005. PMC 6725417. PMID 16014723.
- Schiavon E, Sacco T, Cassulini RR, Gurrola G, Tempia F, Possani LD, Wanke E (July 2006). "Resurgent current and voltage sensor trapping enhanced activation by a beta-scorpion toxin solely in Nav1.6 channel. Significance in mice Purkinje neurons". The Journal of Biological Chemistry. 281 (29): 20326–37. doi:10.1074/jbc.M600565200. PMID 16702217.
- Shirahata E, Iwasaki H, Takagi M, Lin C, Bennett V, Okamura Y, Hayasaka K (September 2006). "Ankyrin-G regulates inactivation gating of the neuronal sodium channel, Nav1.6". Journal of Neurophysiology. 96 (3): 1347–57. doi:10.1152/jn.01264.2005. PMID 16775201.
- Black JA, Newcombe J, Trapp BD, Waxman SG (September 2007). "Sodium channel expression within chronic multiple sclerosis plaques". Journal of Neuropathology and Experimental Neurology. 66 (9): 828–37. doi:10.1097/nen.0b013e3181462841. PMID 17805013.
External links
- "SCN8A Website and Registry". University of Arizona.
- "SCN8A Family Support". The Cute Syndrome Foundation.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.