Nabitan
 | |
| Clinical data | |
|---|---|
| Other names | Nabutam, benzopyranoperidine, SP-106, Abbott 40656 |
| Drug class | Cannabinoid |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
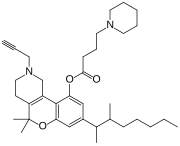
| Formula | C35H52N2O3 |
| Molar mass | 548.812 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Nabitan (nabutam, benzopyranoperidine, SP-106, Abbott 40656) is a synthetic cannabinoid analog of dronabinol (Δ9-tetrahydrocannabinol) and dimethylheptylpyran.[1] It exhibits antiemetic and analgesic effects, most likely by binding to and activating the CB1 and CB2 cannabinoid receptors, and reduced intraocular pressure in animal tests, making it potentially useful in the treatment of glaucoma.[2]
Nabitan has the advantage of being water-soluble, unlike most cannabinoid derivatives, and was researched for potential use as an analgesic or sedative,[3] although it was never developed for clinical use and is not currently used in medicine, as dronabinol or nabilone were felt to be more useful. However, it is sometimes used in research into the potential therapeutic applications of cannabinoids.
See also
References
- ^ Razdan RK. The Total Synthesis of Cannabinoids. Wiley-Interscience 1980
- ^ Razdan RK, Howes JF (1983). "Drugs related to tetrahydrocannabinol". Medicinal Research Reviews. 3 (2): 119–46. doi:10.1002/med.2610030203. PMID 6134882. S2CID 31313909.
- ^ Archer RA (1974). "The cannabinoids: therapeutic potentials". Annual Reports in Medicinal Chemistry. 9: 253–9. doi:10.1016/s0065-7743(08)61448-7. PMID 12307093.
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
