NNE1
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H24N2O |
| Molar mass | 356.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
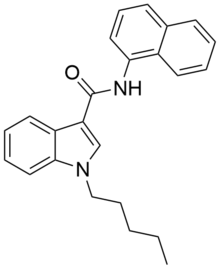
NNE1 (also known as NNEI, MN-24 and AM-6527) is an indole-based synthetic cannabinoid, representing a molecular hybrid of APICA and JWH-018[1] that is an agonist for the cannabinoid receptors, with Ki values of 60.09 nM at CB1 and 45.298 nM at CB2 and EC50 values of 9.481 nM at CB1 and 1.008 nM at CB2.[2] It was invented by Abbott and has a CB1 receptor pEC50 of 8.9 with around 80x selectivity over the related CB2 receptor.[3] It is suspected that metabolic hydrolysis of the amide group of NNE1 may release 1-naphthylamine, a known carcinogen, given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, and NNE1 was banned in New Zealand in 2012 as a temporary class drug to stop it being used as an ingredient in then-legal synthetic cannabis products.[4] NNE1 was subsequently found to be responsible for the death of a man in Japan in 2014.[5]
See also
References
- ^ Uchiyama N, Matsuda S, Kawamura M, Shimokawa Y, Kikura-Hanajiri R, Aritake K, et al. (October 2014). "Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products". Forensic Science International. 243: 1–13. doi:10.1016/j.forsciint.2014.03.013. PMID 24769262.
- ^ Gamage TF, Farquhar CE, Lefever TW, Marusich JA, Kevin RC, McGregor IS, et al. (May 2018). "Molecular and Behavioral Pharmacological Characterization of Abused Synthetic Cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA". The Journal of Pharmacology and Experimental Therapeutics. 365 (2): 437–446. doi:10.1124/jpet.117.246983. PMC 5932312. PMID 29549157.
- ^ Blaazer AR, Lange JH, van der Neut MA, Mulder A, den Boon FS, Werkman TR, et al. (October 2011). "Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: design, synthesis, structure-activity relationships, physicochemical properties and biological activity". European Journal of Medicinal Chemistry. 46 (10): 5086–98. doi:10.1016/j.ejmech.2011.08.021. PMID 21885167.
- ^ New Zealand Government Gazette, Notice Number 7051, 1 November 2012
- ^ Sasaki C, Saito T, Shinozuka T, Irie W, Murakami C, Maeda K, et al. (January 2015). "A case of death caused by abuse of a synthetic cannabinoid N -1-naphthalenyl-1-pentyl-1H -indole-3-carboxamide". Forensic Toxicology. 33 (1): 165–169. doi:10.1007/s11419-014-0246-5. S2CID 13524298.
