Helium-3
 | |
| General | |
|---|---|
| Symbol | 3He |
| Names | helium-3, 3He, He-3, tralphium (obsolete) |
| Protons (Z) | 2 |
| Neutrons (N) | 1 |
| Nuclide data | |
| Natural abundance | 0.000137% (% He on Earth) 0.001% (% He in Solar System) |
| Half-life (t1/2) | stable |
| Isotope mass | 3.0160293 Da |
| Spin | 1⁄2 |
| Parent isotopes | 3H (beta decay of tritium) |
| Isotopes of helium Complete table of nuclides | |
Helium-3 (3He[1][2] see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and protium (ordinary hydrogen) are the only stable nuclides with more protons than neutrons. It was discovered in 1939.
Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. It is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some found in the terrestrial atmosphere is a remnant of atmospheric and underwater nuclear weapons testing.
Nuclear fusion using helium-3 has long been viewed as a desirable future energy source. The fusion of two of its atoms would be aneutronic, not release the dangerous radiation of traditional fusion or require much higher temperatures.[3] The process may unavoidably create other reactions that themselves would cause the surrounding material to become radioactive.[4]
Helium-3 is thought to be more abundant on the Moon than on Earth, having been deposited in the upper layer of regolith by the solar wind over billions of years,[5] though still lower in abundance than in the Solar System's gas giants.[6][7]
History
The existence of helium-3 was first proposed in 1934 by the Australian nuclear physicist Mark Oliphant while he was working at the University of Cambridge Cavendish Laboratory. Oliphant had performed experiments in which fast deuterons collided with deuteron targets (incidentally, the first demonstration of nuclear fusion).[8] Isolation of helium-3 was first accomplished by Luis Alvarez and Robert Cornog in 1939.[9][10] Helium-3 was thought to be a radioactive isotope until it was also found in samples of natural helium, which is mostly helium-4, taken both from the terrestrial atmosphere and from natural gas wells.[11]
Physical properties
Due to its low atomic mass of 3.016 u, helium-3 has some physical properties different from those of helium-4, with a mass of 4.0026 u. On account of the weak, induced dipole–dipole interaction between the helium atoms, their microscopic physical properties are mainly determined by their zero-point energy. Also, the microscopic properties of helium-3 cause it to have a higher zero-point energy than helium-4. This implies that helium-3 can overcome dipole–dipole interactions with less thermal energy than helium-4 can.
The quantum mechanical effects on helium-3 and helium-4 are significantly different because with two protons, two neutrons, and two electrons, helium-4 has an overall spin of zero, making it a boson, but with one fewer neutron, helium-3 has an overall spin of one half, making it a fermion.
Pure helium-3 gas boils at 3.19 K compared with helium-4 at 4.23 K, and its critical point is also lower at 3.35 K, compared with helium-4 at 5.2 K. Helium-3 has less than half the density of helium-4 when it is at its boiling point: 59 g/L compared to 125 g/L of helium-4 at a pressure of one atmosphere. Its latent heat of vaporization is also considerably lower at 0.026 kJ/mol compared with the 0.0829 kJ/mol of helium-4.[12][13]
Superfluidity

An important property of helium-3, which distinguishes it from the more common helium-4, is that its nucleus is a fermion since it contains an odd number of spin 1⁄2 particles. Helium-4 nuclei are bosons, containing an even number of spin 1⁄2 particles. This is a direct result of the addition rules for quantized angular momentum. At low temperatures (about 2.17 K), helium-4 undergoes a phase transition: A fraction of it enters a superfluid phase that can be roughly understood as a type of Bose–Einstein condensate. Such a mechanism is not available for helium-3 atoms, which are fermions. Many speculated that helium-3 could also become a superfluid at much lower temperatures, if the atoms formed into pairs analogous to Cooper pairs in the BCS theory of superconductivity. Each Cooper pair, having integer spin, can be thought of as a boson. During the 1970s, David Lee, Douglas Osheroff and Robert Coleman Richardson discovered two phase transitions along the melting curve, which were soon realized to be the two superfluid phases of helium-3.[14][15] The transition to a superfluid occurs at 2.491 millikelvins on the melting curve. They were awarded the 1996 Nobel Prize in Physics for their discovery. Alexei Abrikosov, Vitaly Ginzburg, and Tony Leggett won the 2003 Nobel Prize in Physics for their work on refining understanding of the superfluid phase of helium-3.[16]
In a zero magnetic field, there are two distinct superfluid phases of 3He, the A-phase and the B-phase. The B-phase is the low-temperature, low-pressure phase which has an isotropic energy gap. The A-phase is the higher temperature, higher pressure phase that is further stabilized by a magnetic field and has two point nodes in its gap. The presence of two phases is a clear indication that 3He is an unconventional superfluid (superconductor), since the presence of two phases requires an additional symmetry, other than gauge symmetry, to be broken. In fact, it is a p-wave superfluid, with spin one, S=1, and angular momentum one, L=1. The ground state corresponds to total angular momentum zero, J=S+L=0 (vector addition). Excited states are possible with non-zero total angular momentum, J>0, which are excited pair collective modes. Because of the extreme purity of superfluid 3He (since all materials except 4He have solidified and sunk to the bottom of the liquid 3He and any 4He has phase separated entirely, this is the most pure condensed matter state), these collective modes have been studied with much greater precision than in any other unconventional pairing system.
Natural abundance
Terrestrial abundance
3He is a primordial substance in the Earth's mantle, thought to have become entrapped in the Earth during planetary formation. The ratio of 3He to 4He within the Earth's crust and mantle is less than that of estimates of solar disk composition as obtained from meteorite and lunar samples, with terrestrial materials generally containing lower 3He/4He ratios due to production of 4He from radioactive decay.
3He has a cosmological ratio of 300 atoms per million atoms of 4He (at. ppm),[17] leading to the assumption that the original ratio of these primordial gases in the mantle was around 200-300 ppm when Earth was formed. Over Earth's history alpha-particle decay of uranium, thorium and other radioactive isotopes has generated significant amounts of 4He, such that only around 7% of the helium now in the mantle is primordial helium,[17] lowering the total 3He/4He ratio to around 20 ppm. Ratios of 3He/4He in excess of atmospheric are indicative of a contribution of 3He from the mantle. Crustal sources are dominated by the 4He produced by radioactive decay.
The ratio of helium-3 to helium-4 in natural Earth-bound sources varies greatly.[18][19] Samples of the lithium ore spodumene from Edison Mine, South Dakota were found to contain 12 parts of helium-3 to a million parts of helium-4. Samples from other mines showed 2 parts per million.[18]
Helium is also present as up to 7% of some natural gas sources,[20] and large sources have over 0.5% (above 0.2% makes it viable to extract).[21] The fraction of 3He in helium separated from natural gas in the U.S. was found to range from 70 to 242 parts per billion.[22][23] Hence the US 2002 stockpile of 1 billion normal m3[21] would have contained about 12 to 43 kilograms (26 to 95 lb) of helium-3. According to American physicist Richard Garwin, about 26 cubic metres (920 cu ft) or almost 5 kilograms (11 lb) of 3He is available annually for separation from the US natural gas stream. If the process of separating out the 3He could employ as feedstock the liquefied helium typically used to transport and store bulk quantities, estimates for the incremental energy cost range from $34 to $300 per litre ($150 to $1,360/imp gal) NTP, excluding the cost of infrastructure and equipment.[22] Algeria's annual gas production is assumed to contain 100 million normal cubic metres[21] and this would contain between 7 and 24 cubic metres (250 and 850 cu ft) of helium-3 (about 1 to 4 kilograms (2.2 to 8.8 lb)) assuming a similar 3He fraction.
3He is also present in the Earth's atmosphere. The natural abundance of 3He in naturally occurring helium gas is 1.38×10−6 (1.38 parts per million). The partial pressure of helium in the Earth's atmosphere is about 0.52 pascals (7.5×10−5 psi), and thus helium accounts for 5.2 parts per million of the total pressure (101325 Pa) in the Earth's atmosphere, and 3He thus accounts for 7.2 parts per trillion of the atmosphere. Since the atmosphere of the Earth has a mass of about 5.14×1018 kilograms (1.133×1019 lb),[24] the mass of 3He in the Earth's atmosphere is the product of these numbers, or about 37,000 tonnes (36,000 long tons; 41,000 short tons) of 3He. (In fact the effective figure is ten times smaller, since the above ppm are ppmv and not ppmw. One must multiply by 3 (the molecular mass of helium-3) and divide by 29 (the mean molecular mass of the atmosphere), resulting in 3,828 tonnes (3,768 long tons; 4,220 short tons) of helium-3 in the earth's atmosphere.)
3He is produced on Earth from three sources: lithium spallation, cosmic rays, and beta decay of tritium (3H). The contribution from cosmic rays is negligible within all except the oldest regolith materials, and lithium spallation reactions are a lesser contributor than the production of 4He by alpha particle emissions.
The total amount of helium-3 in the mantle may be in the range of 0.1–1 megatonne (98,000–984,000 long tons; 110,000–1,100,000 short tons). Most mantle is not directly accessible. Some helium-3 leaks up through deep-sourced hotspot volcanoes such as those of the Hawaiian Islands, but only 300 grams (11 oz) per year is emitted to the atmosphere. Mid-ocean ridges emit another 3 kilograms per year (8.2 g/d). Around subduction zones, various sources produce helium-3 in natural gas deposits which possibly contain a thousand tonnes of helium-3 (although there may be 25 thousand tonnes if all ancient subduction zones have such deposits). Wittenberg estimated that United States crustal natural gas sources may have only half a tonne total.[25] Wittenberg cited Anderson's estimate of another 1,200 tonnes (1,200 long tons; 1,300 short tons) in interplanetary dust particles on the ocean floors.[26] In the 1994 study, extracting helium-3 from these sources consumes more energy than fusion would release.[27]
Lunar surface
See Extraterrestrial mining or Lunar resources
Solar nebula (primordial) abundance
One early estimate of the primordial ratio of 3He to 4He in the solar nebula has been the measurement of their ratio in the atmosphere of Jupiter, measured by the mass spectrometer of the Galileo atmospheric entry probe. This ratio is about 1:10,000,[28] or 100 parts of 3He per million parts of 4He. This is roughly the same ratio of the isotopes as in lunar regolith, which contains 28 ppm helium-4 and 2.8 ppb helium-3 (which is at the lower end of actual sample measurements, which vary from about 1.4 to 15 ppb). Terrestrial ratios of the isotopes are lower by a factor of 100, mainly due to enrichment of helium-4 stocks in the mantle by billions of years of alpha decay from uranium, thorium as well as their decay products and extinct radionuclides.
Human production
Tritium decay
Virtually all helium-3 used in industry today is produced from the radioactive decay of tritium, given its very low natural abundance and its very high cost.
Production, sales and distribution of helium-3 in the United States are managed by the US Department of Energy (DOE) DOE Isotope Program.[29]
While tritium has several different experimentally determined values of its half-life, NIST lists 4,500±8 d (12.32±0.02 years).[30] It decays into helium-3 by beta decay as in this nuclear equation:
Among the total released energy of 18.6 keV, the part taken by electron's kinetic energy varies, with an average of 5.7 keV, while the remaining energy is carried off by the nearly undetectable electron antineutrino. Beta particles from tritium can penetrate only about 6.0 millimetres (0.24 in) of air, and they are incapable of passing through the dead outermost layer of human skin.[31] The unusually low energy released in the tritium beta decay makes the decay (along with that of rhenium-187) appropriate for absolute neutrino mass measurements in the laboratory (the most recent experiment being KATRIN).
The low energy of tritium's radiation makes it difficult to detect tritium-labeled compounds except by using liquid scintillation counting.
Tritium is a radioactive isotope of hydrogen and is typically produced by bombarding lithium-6 with neutrons in a nuclear reactor. The lithium nucleus absorbs a neutron and splits into helium-4 and tritium. Tritium decays into helium-3 with a half-life of 12.3 years, so helium-3 can be produced by simply storing the tritium until it undergoes radioactive decay. As tritium forms a stable compound with oxygen (tritiated water) while helium-3 does not, the storage and collection process could continuously collect the material that outgasses from the stored material.
Tritium is a critical component of nuclear weapons and historically it was produced and stockpiled primarily for this application. The decay of tritium into helium-3 reduces the explosive power of the fusion warhead, so periodically the accumulated helium-3 must be removed from warhead reservoirs and tritium in storage. Helium-3 removed during this process is marketed for other applications.
For decades this has been, and remains, the principal source of the world's helium-3.[32] Since the signing of the START I Treaty in 1991 the number of nuclear warheads that are kept ready for use has decreased.[33][34] This has reduced the quantity of helium-3 available from this source. Helium-3 stockpiles have been further diminished by increased demand,[22] primarily for use in neutron radiation detectors and medical diagnostic procedures. US industrial demand for helium-3 reached a peak of 70,000 litres (15,000 imp gal; 18,000 US gal) (approximately 8 kilograms (18 lb)) per year in 2008. Price at auction, historically about $100 per litre ($450/imp gal), reached as high as $2,000 per litre ($9,100/imp gal).[35] Since then, demand for helium-3 has declined to about 6,000 litres (1,300 imp gal; 1,600 US gal) per year due to the high cost and efforts by the DOE to recycle it and find substitutes. Assuming a density of 114 grams per cubic metre (0.192 lb/cu yd) at $100/l helium-3 would be about a thirtieth as expensive as tritium (roughly $880 per gram ($25,000/oz) vs roughly $30,000 per gram ($850,000/oz)) while at $2000/l helium-3 would be about half as expensive as tritium ($17,540 per gram ($497,000/oz) vs $30,000 per gram ($850,000/oz)).
The DOE recognized the developing shortage of both tritium and helium-3, and began producing tritium by lithium irradiation at the Tennessee Valley Authority's Watts Bar Nuclear Generating Station in 2010.[22] In this process tritium-producing burnable absorber rods (TPBARs) containing lithium in a ceramic form are inserted into the reactor in place of the normal boron control rods[36] Periodically the TPBARs are replaced and the tritium extracted.
Currently only two commercial nuclear reactors (Watts Bar Nuclear Plant Units 1 and 2) are being used for tritium production but the process could, if necessary, be vastly scaled up to meet any conceivable demand simply by utilizing more of the nation's power reactors[citation needed]. Substantial quantities of tritium and helium-3 could also be extracted from the heavy water moderator in CANDU nuclear reactors.[22][37] India and Canada, the two countries with the largest heavy water reactor fleet, are both known to extract tritium from moderator/coolant heavy water, but those amounts are not nearly enough to satisfy global demand of either tritium or helium-3.
As tritium is also produced inadvertently in various processes in light water reactors (see the article on tritium for details), extraction from those sources could be another source of helium-3. If the annual discharge of tritium (per 2018 figures) at La Hague reprocessing facility is taken as a basis, the amounts discharged (31.2 grams (1.10 oz) at La Hague) are not nearly enough to satisfy demand, even if 100% recovery is achieved.
Uses
Helium-3 spin echo
Helium-3 can be used to do spin echo experiments of surface dynamics, which are underway at the Surface Physics Group at the Cavendish Laboratory in Cambridge and in the Chemistry Department at Swansea University.
Neutron detection
Helium-3 is an important isotope in instrumentation for neutron detection. It has a high absorption cross section for thermal neutron beams and is used as a converter gas in neutron detectors. The neutron is converted through the nuclear reaction
- n + 3He → 3H + 1H + 0.764 MeV
into charged particles tritium ions (T, 3H) and Hydrogen ions, or protons (p, 1H) which then are detected by creating a charge cloud in the stopping gas of a proportional counter or a Geiger–Müller tube.[40]
Furthermore, the absorption process is strongly spin-dependent, which allows a spin-polarized helium-3 volume to transmit neutrons with one spin component while absorbing the other. This effect is employed in neutron polarization analysis, a technique which probes for magnetic properties of matter.[41][42][43][44]
The United States Department of Homeland Security had hoped to deploy detectors to spot smuggled plutonium in shipping containers by their neutron emissions, but the worldwide shortage of helium-3 following the drawdown in nuclear weapons production since the Cold War has to some extent prevented this.[45] As of 2012, DHS determined the commercial supply of boron-10 would support converting its neutron detection infrastructure to that technology.[46]
Cryogenics
A helium-3 refrigerator uses helium-3 to achieve temperatures of 0.2 to 0.3 kelvin. A dilution refrigerator uses a mixture of helium-3 and helium-4 to reach cryogenic temperatures as low as a few thousandths of a kelvin.[47]
Medical imaging
Helium-3 nuclei have an intrinsic nuclear spin of 1⁄2, and a relatively high magnetogyric ratio. Helium-3 can be hyperpolarized using non-equilibrium means such as spin-exchange optical pumping.[48] During this process, circularly polarized infrared laser light, tuned to the appropriate wavelength, is used to excite electrons in an alkali metal, such as caesium or rubidium inside a sealed glass vessel. The angular momentum is transferred from the alkali metal electrons to the noble gas nuclei through collisions. In essence, this process effectively aligns the nuclear spins with the magnetic field in order to enhance the NMR signal. The hyperpolarized gas may then be stored at pressures of 10 atm, for up to 100 hours. Following inhalation, gas mixtures containing the hyperpolarized helium-3 gas can be imaged with an MRI scanner to produce anatomical and functional images of lung ventilation. This technique is also able to produce images of the airway tree, locate unventilated defects, measure the alveolar oxygen partial pressure, and measure the ventilation/perfusion ratio. This technique may be critical for the diagnosis and treatment management of chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD), emphysema, cystic fibrosis, and asthma.[49]
Radio energy absorber for tokamak plasma experiments
Both MIT's Alcator C-Mod tokamak and the Joint European Torus (JET) have experimented with adding a little helium-3 to a H–D plasma to increase the absorption of radio-frequency (RF) energy to heat the hydrogen and deuterium ions, a "three-ion" effect.[50][51]
Nuclear fuel
| Reactants | Products | Q | n/MeV | |
|---|---|---|---|---|
| First-generation fusion fuels | 2D + 2D | 3He + 1 0n |
3.268 MeV | 0.306 |
| 2D + 2D | 3T + 1 1p |
4.032 MeV | 0 | |
| 2D + 3T | 4He + 1 0n |
17.571 MeV | 0.057 | |
| Second-generation fusion fuel | 2D + 3He | 4He + 1 1p |
18.354 MeV | 0 |
| Net result of 2D burning (sum of first 4 rows) | 6 2D | 2(4He + n + p) | 43.225 MeV | 0.046 |
| Third-generation fusion fuels | 3He + 3He | 4He + 2 1 1p |
12.86 MeV | 0 |
| 11B + 1 1p |
3 4He | 8.68 MeV | 0 | |
| Current nuclear fuel | 235U + n | 2 FP+ 2.5n | ~200 MeV | 0.0075 |
3He can be produced by the low temperature fusion of → 3He + γ + 4.98 MeV. If the fusion temperature is below that for the helium nuclei to fuse, the reaction produces a high energy alpha particle which quickly acquires an electron producing a stable light helium ion which can be utilized directly as a source of electricity without producing dangerous neutrons.
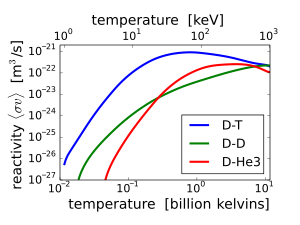
3He can be used in fusion reactions by either of the reactions 2H + 3He → 4He + 1p + 18.3 MeV, or 3He + 3He → 4He + 2 1p + 12.86 MeV.
The conventional deuterium + tritium ("D–T") fusion process produces energetic neutrons which render reactor components radioactive with activation products. The appeal of helium-3 fusion stems from the aneutronic nature of its reaction products. Helium-3 itself is non-radioactive. The lone high-energy by-product, the proton, can be contained by means of electric and magnetic fields. The momentum energy of this proton (created in the fusion process) will interact with the containing electromagnetic field, resulting in direct net electricity generation.[57]
Because of the higher Coulomb barrier, the temperatures required for 2H + 3He fusion are much higher than those of conventional D–T fusion. Moreover, since both reactants need to be mixed together to fuse, reactions between nuclei of the same reactant will occur, and the D–D reaction (2H + 2H) does produce a neutron. Reaction rates vary with temperature, but the D–3He reaction rate is never greater than 3.56 times the D–D reaction rate (see graph). Therefore, fusion using D–3He fuel at the right temperature and a D-lean fuel mixture, can produce a much lower neutron flux than D–T fusion, but is not clean, negating some of its main attraction.
The second possibility, fusing 3He with itself (3He + 3He), requires even higher temperatures (since now both reactants have a +2 charge), and thus is even more difficult than the D-3He reaction. It offers a theoretical reaction that produces no neutrons; the charged protons produced can be contained in electric and magnetic fields, which in turn directly generates electricity. 3He + 3He fusion is feasible as demonstrated in the laboratory and has immense advantages, but commercial viability is many years in the future.[58]
The amounts of helium-3 needed as a replacement for conventional fuels are substantial by comparison to amounts currently available. The total amount of energy produced in the 2D + 3He reaction is 18.4 MeV, which corresponds to some 493 megawatt-hours (4.93×108 W·h) per three grams (one mole) of 3He. If the total amount of energy could be converted to electrical power with 100% efficiency (a physical impossibility), it would correspond to about 30 minutes of output of a gigawatt electrical plant per mole of 3He. Thus, a year's production (at 6 grams for each operation hour) would require 52.5 kilograms of helium-3. The amount of fuel needed for large-scale applications can also be put in terms of total consumption: electricity consumption by 107 million U.S. households in 2001[59] totaled 1,140 billion kW·h (1.14×1015 W·h). Again assuming 100% conversion efficiency, 6.7 tonnes per year of helium-3 would be required for that segment of the energy demand of the United States, 15 to 20 tonnes per year given a more realistic end-to-end conversion efficiency.[citation needed]
A second-generation approach to controlled fusion power involves combining helium-3 and deuterium, 2D. This reaction produces an alpha particle and a high-energy proton. The most important potential advantage of this fusion reaction for power production as well as other applications lies in its compatibility with the use of electrostatic fields to control fuel ions and the fusion protons. High speed protons, as positively charged particles, can have their kinetic energy converted directly into electricity, through use of solid-state conversion materials as well as other techniques. Potential conversion efficiencies of 70% may be possible, as there is no need to convert proton energy to heat in order to drive a turbine-powered electrical generator.[citation needed]
He-3 power plants
There have been many claims about the capabilities of helium-3 power plants. According to proponents, fusion power plants operating on deuterium and helium-3 would offer lower capital and operating costs than their competitors due to less technical complexity, higher conversion efficiency, smaller size, the absence of radioactive fuel, no air or water pollution, and only low-level radioactive waste disposal requirements. Recent estimates suggest that about $6 billion in investment capital will be required to develop and construct the first helium-3 fusion power plant. Financial break even at today's wholesale electricity prices (5 US cents per kilowatt-hour) would occur after five 1-gigawatt plants were on line, replacing old conventional plants or meeting new demand.[60]
The reality is not so clear-cut. The most advanced fusion programs in the world are inertial confinement fusion (such as National Ignition Facility) and magnetic confinement fusion (such as ITER and Wendelstein 7-X). In the case of the former, there is no solid roadmap to power generation. In the case of the latter, commercial power generation is not expected until around 2050.[61] In both cases, the type of fusion discussed is the simplest: D–T fusion. The reason for this is the very low Coulomb barrier for this reaction; for D+3He, the barrier is much higher, and it is even higher for 3He–3He. The immense cost of reactors like ITER and National Ignition Facility are largely due to their immense size, yet to scale up to higher plasma temperatures would require reactors far larger still. The 14.7 MeV proton and 3.6 MeV alpha particle from D–3He fusion, plus the higher conversion efficiency, means that more electricity is obtained per kilogram than with D–T fusion (17.6 MeV), but not that much more. As a further downside, the rates of reaction for helium-3 fusion reactions are not particularly high, requiring a reactor that is larger still or more reactors to produce the same amount of electricity.
In 2022, Helion Energy claimed that their 7th fusion prototype (Polaris; fully funded and under construction as of September 2022) will demonstrate "net electricity from fusion", and will demonstrate "helium-3 production through deuterium–deuterium fusion" by means of a "patented high-efficiency closed-fuel cycle".[62]
Alternatives to He-3
To attempt to work around this problem of massively large power plants that may not even be economical with D–T fusion, let alone the far more challenging D–3He fusion, a number of other reactors have been proposed – the Fusor, Polywell, Focus fusion, and many more, though many of these concepts have fundamental problems with achieving a net energy gain, and generally attempt to achieve fusion in thermal disequilibrium, something that could potentially prove impossible,[63] and consequently, these long-shot programs tend to have trouble garnering funding despite their low budgets. Unlike the "big" and "hot" fusion systems, if such systems worked, they could scale to the higher barrier aneutronic fuels, and so their proponents tend to promote p-B fusion, which requires no exotic fuel such as helium-3.
Extraterrestrial
Moon
Materials on the Moon's surface contain helium-3 at concentrations between 1.4 and 15 ppb in sunlit areas,[64][65] and may contain concentrations as much as 50 ppb in permanently shadowed regions.[7] A number of people, starting with Gerald Kulcinski in 1986,[66] have proposed to explore the Moon, mine lunar regolith and use the helium-3 for fusion. Because of the low concentrations of helium-3, any mining equipment would need to process extremely large amounts of regolith (over 150 tonnes of regolith to obtain one gram of helium-3).[67]
The primary objective of Indian Space Research Organisation's first lunar probe called Chandrayaan-1, launched on October 22, 2008, was reported in some sources to be mapping the Moon's surface for helium-3-containing minerals.[68] No such objective is mentioned in the project's official list of goals, though many of its scientific payloads have held helium-3-related applications.[69][70]
Cosmochemist and geochemist Ouyang Ziyuan from the Chinese Academy of Sciences who is now in charge of the Chinese Lunar Exploration Program has already stated on many occasions that one of the main goals of the program would be the mining of helium-3, from which operation "each year, three space shuttle missions could bring enough fuel for all human beings across the world".[71]
In January 2006, the Russian space company RKK Energiya announced that it considers lunar helium-3 a potential economic resource to be mined by 2020,[72] if funding can be found.[73][74]
Not all writers feel the extraction of lunar helium-3 is feasible, or even that there will be a demand for it for fusion. Dwayne Day, writing in The Space Review in 2015, characterises helium-3 extraction from the Moon for use in fusion as magical thinking about an unproven technology, and questions the feasibility of lunar extraction, as compared to production on Earth.[75]
Gas giants
Mining gas giants for helium-3 has also been proposed.[76] The British Interplanetary Society's hypothetical Project Daedalus interstellar probe design was fueled by helium-3 mines in the atmosphere of Jupiter, for example.
See also
Notes and references
- ^ Galli, D. (September 2004). "The cosmic saga of 3He". arXiv:astro-ph/0412380v1.
- ^ Ley, Willy (October 1966). "The Delayed Discovery". For Your Information. Galaxy Science Fiction. pp. 116–127.
- ^ Matson, John (12 Jun 2009). "Is MOON's Sci-Fi Vision of Lunar Helium 3 Mining Based in Reality?". Scientific American – News Blog. Archived from the original on 30 August 2017. Retrieved 29 Aug 2017.
- ^ Close, Frank (August 2007). "Fears Over Factoids" (PDF). CERN Document Server. Physicsworld.com. Archived (PDF) from the original on 22 October 2017. Retrieved 8 July 2018.
- ^ Fa WenZhe; Jin YaQiu (December 2010). "Global inventory of Helium-3 in lunar regoliths estimated by a multi-channel microwave radiometer on the Chang-E 1 lunar satellite". Archived from the original on 2017-10-11. Retrieved 2012-12-12.
- ^ Slyuta, E. N.; Abdrakhimov, A. M.; Galimov, E. M. (March 12–16, 2007). The Estimation of Helium-3 Probable Reserves in Lunar Regolith (PDF). 38th Lunar and Planetary Science Conference. p. 2175. Archived (PDF) from the original on 2008-07-05. Retrieved 2007-05-31.
- ^ a b Cocks, F. H. (2010). "3He in permanently shadowed lunar polar surfaces". Icarus. 206 (2): 778–779. Bibcode:2010Icar..206..778C. doi:10.1016/j.icarus.2009.12.032.
- ^ Oliphant, M. L. E.; Harteck, P.; Rutherford, E. (1934). "Transmutation Effects Observed with Heavy Hydrogen". Proceedings of the Royal Society A. 144 (853): 692–703. Bibcode:1934RSPSA.144..692O. doi:10.1098/rspa.1934.0077. JSTOR 2935553.
- ^ Alvarez, Luis; Cornog, Robert (1939). "Helium and Hydrogen of Mass 3". Physical Review. 56 (6): 613. Bibcode:1939PhRv...56..613A. doi:10.1103/PhysRev.56.613.
- ^ Alvarez, Luis W; Peter Trower, W (1987). Discovering Alvarez: selected works of Luis W. Alvarez, with commentary by his students and colleagues. University of Chicago Press. pp. 26–30. ISBN 978-0-226-81304-2.
- ^ "Lawrence and His Laboratory: Episode: A Productive Error". Newsmagazine Publication. 1981. Archived from the original on 2017-05-10. Retrieved 2009-09-01.
- ^ Teragon's Summary of Cryogen Properties Archived 2017-08-09 at the Wayback Machine Teragon Research, 2005
- ^ Chase, C. E.; Zimmerman, G. O. (1973). "Measurements of P-V-T and Critical Indices of He3". Journal of Low Temperature Physics. 11 (5–6): 551. Bibcode:1973JLTP...11..551C. doi:10.1007/BF00654447. S2CID 123038029.
- ^ Osheroff, D. D.; Richardson, R. C.; Lee, D. M. (1972). "Evidence for a New Phase of Solid He3". Physical Review Letters. 28 (14): 885–888. Bibcode:1972PhRvL..28..885O. doi:10.1103/PhysRevLett.28.885.
- ^ Osheroff, D. D.; Gully, W. J.; Richardson, R. C.; Lee, D. M. (1972). "New Magnetic Phenomena in Liquid He3 below 3 mK". Physical Review Letters. 29 (14): 920–923. Bibcode:1972PhRvL..29..920O. doi:10.1103/PhysRevLett.29.920.
- ^ Leggett, A. J. (1972). "Interpretation of Recent Results on He3 below 3 mK: A New Liquid Phase?". Physical Review Letters. 29 (18): 1227–1230. Bibcode:1972PhRvL..29.1227L. doi:10.1103/PhysRevLett.29.1227.
- ^ a b Wittenberg 1994
- ^ a b Aldrich, L.T.; Nier, Alfred O. Phys. Rev. 74, 1590 – 1594 (1948). The Occurrence of He3 in Natural Sources of Helium. Page 1592, Tables I and II.
- ^ Holden, Normen E. 1993. Helium Isotopic Abundance Variation in Nature. copy of paper BNL-49331 "Table II. 3He Abundance of Natural Gas ... 3He in ppm ... Aldrich 0.05 – 0.5 ... Sano 0.46 – 22.7", "Table V. ... of Water ... 3He in ppm ... 1.6 – 1.8 East Pacific ... 0.006 – 1.5 Manitoba Chalk River ... 164 Japan Sea" (Aldrich measured Helium from US wells, Sano that of Taiwan gas: Sano, Yuji; Wakita, Hiroshi; Huang, Chin-Wang (September 1986). "Helium flux in a continental land area estimated from 3He/4He ratio in northern Taiwan". Nature. 323 (6083): 55–57. Bibcode:1986Natur.323...55S. doi:10.1038/323055a0. ISSN 1476-4687. S2CID 4358031.)
- ^ WebElements Periodic Table: Professional Edition: Helium: key information Archived 2008-05-09 at the Wayback Machine. Webelements.com. Retrieved on 2011-11-08.
- ^ a b c Smith, D.M. "any concentration of helium above approximately 0.2 percent is considered worthwhile examining" ... "U.S. government still owns approximately 1 billion nm3 of helium inventory", "Middle East and North Africa ... many very large, helium-rich (up to 0.5 percent) natural gas fields" (Smith uses nm3 to mean "normal cubic metre", elsewhere called "cubic metre at NTP)
- ^ a b c d e Shea, Dana A.; Morgan, Daniel (22 December 2010). The Helium-3 Shortage: Supply, Demand, and Options for Congress (PDF) (Report). Congressional Research Service. 7-5700. Archived (PDF) from the original on 4 March 2016. Retrieved 23 December 2015.
- ^ Davidson, Thomas A.; Emerson, David E. (1990). Method and Apparatus for Direct Determination of Helium-3 in Natural Gas and Helium (Report). Bureau of Mines, US Department of the Interior. Report of Investigations 9302.
- ^ Smith, Lesley; Trenberth, Kevin E. (2005). "The Mass of the Atmosphere: A Constraint on Global Analyses". Journal of Climate. 18 (6): 864–875. Bibcode:2005JCli...18..864T. doi:10.1175/JCLI-3299.1. S2CID 16754900.
- ^ Wittenberg 1994 p. 3, Table 1; p. 9.
- ^ Wittenberg 1994 Page A-1 citing Anderson 1993, "1200 metric tonne"
- ^ Wittenberg 1994 Page A-4 "1 kg (3He), pumping power would be 1.13×106 MWyr ... fusion power derived ... 19 MWyr"
- ^ Niemann, Hasso B.; Atreya, Sushil K.; Carignan, George R.; Donahue, Thomas M.; Haberman, John A.; Harpold, Dan N.; Hartle, Richard E.; Hunten, Donald M.; et al. (1996). "The Galileo Probe Mass Spectrometer: Composition of Jupiter's Atmosphere". Science. 272 (5263): 846–9. Bibcode:1996Sci...272..846N. doi:10.1126/science.272.5263.846. PMID 8629016. S2CID 3242002.
- ^ "Isotope Development & Production for Research and Applications (IDPRA)". US Department of Energy Office of Science. 18 October 2018. Archived from the original on 19 October 2011. Retrieved 11 January 2019.
- ^ Lucas, L. L. & Unterweger, M. P. (2000). "Comprehensive Review and Critical Evaluation of the Half-Life of Tritium". Journal of Research of the National Institute of Standards and Technology. 105 (4): 541–549. doi:10.6028/jres.105.043. PMC 4877155. PMID 27551621.
- ^ Nuclide safety data sheet: Hydrogen-3. ehso.emory.edu
- ^ "Savannah River Tritium Enterprise: Fact Sheet" (PDF). Archived (PDF) from the original on 2016-12-22. Retrieved 2016-03-01.
- ^ Charmian Schaller Accelerator Production of Tritium – That Could Mean 40 Years of Work. Los Alamos Monitor. March 1, 1998
- ^ Science for Democratic Action Vol. 5 No. 1 Archived 2006-09-27 at the Wayback Machine. IEER. Retrieved on 2011-11-08;
- ^ Physics Projects Deflate for Lack of Helium-3 . Spectrum.ieee.org. Retrieved on 2011-11-08.
- ^ Tritium Production Archived 2016-08-27 at the Wayback Machine Nuclear Regulatory Commission, 2005.
- ^ CA 2810716, Sur, Bhaskar; Rodrigo, Lakshman & Didsbury, Richard, "System and method for collecting 3He gas from heavy water nuclear reactors", published 30 September 2013, issued 2013 Archived 23 December 2015 at the Wayback Machine
- ^ "Basic policy on handling of the ALPS treated water" (PDF). Ministry of Economy, Trade and Industry. 13 April 2021.
- ^ "2020년도 원전주변 환경방사능 조사 및 평가보고서" [2020 Environmental Radiation Survey and Evaluation Report Around Nuclear Power Plant]. Korea Hydro & Nuclear Power. 26 April 2021. p. 25. (table 8)
- ^ A Modular Neutron Detector | Summer 2003| Los Alamos National Laboratory Archived 2008-05-03 at the Wayback Machine. Lanl.gov. Retrieved on 2011-11-08.
- ^ NCNR Neutron Spin Filters Archived 2007-05-20 at the Wayback Machine. Ncnr.nist.gov (2004-04-28). Retrieved on 2011-11-08.
- ^ 3He-spin-filters/ ILL 3He spin filters[permanent dead link]. Ill.eu (2010-10-22). Retrieved on 2011-11-08.
- ^ Gentile, T.R.; Jones, G.L.; Thompson, A.K.; Barker, J.; Glinka, C.J.; Hammouda, B.; Lynn, J.W. (2000). "SANS polarization analysis with nuclear spin-polarized 3He" (PDF). J. Appl. Crystallogr. 33 (3): 771–774. Bibcode:2000JApCr..33..771G. doi:10.1107/S0021889800099817. Archived (PDF) from the original on 2012-04-02. Retrieved 2011-11-08.
- ^ Neutron Spin Filters: Polarized 3He Archived 2011-10-16 at the Wayback Machine. NIST.gov
- ^ Wald, Matthew L.. (2009-11-22) 3Helium.html?partner=rss&emc=rss Nuclear Bomb Detectors Stopped by Material Shortage. Nytimes.com. Retrieved on 2011-11-08.
- ^ "Office of Science" (PDF). Archived from the original (PDF) on 2014-07-26. Retrieved 2014-07-18.
- ^ Dilution Refrigeration. cern.ch
- ^ Leawoods, Jason C.; Yablonskiy, Dmitriy A.; Saam, Brian; Gierada, David S.; Conradi, Mark S. (2001). "Hyperpolarized 3He Gas Production and MR Imaging of the Lung". Concepts in Magnetic Resonance. 13 (5): 277–293. CiteSeerX 10.1.1.492.8128. doi:10.1002/cmr.1014.
- ^ Altes, Talissa; Salerno, Michael (2004). "Hyperpolarized Gas Imaging of the Lung". J Thorac Imaging. 19 (4): 250–258. doi:10.1097/01.rti.0000142837.52729.38. PMID 15502612.
- ^ "MIT Achieves Breakthrough in Nuclear Fusion Aug 2017". Archived from the original on 2020-08-01. Retrieved 2020-07-18.
- ^ Kazakov, Ye. O.; et al. (19 June 2017). "Efficient generation of energetic ions in multi-ion plasmas by radio-frequency heating". Nature Physics. 13 (10): 973–978. Bibcode:2017NatPh..13..973K. doi:10.1038/nphys4167. hdl:1721.1/114949. S2CID 106402331. Archived from the original on 1 August 2020. Retrieved 18 July 2020.
- ^ "Inertial Electrostatic Confinement Fusion". Archived from the original on 2021-01-26. Retrieved 2007-05-06.
- ^ "Nuclear Fission and Fusion". Archived from the original on 2007-04-04. Retrieved 2007-05-06.
- ^ "The Fusion Reaction". Archived from the original on 2013-07-31. Retrieved 2007-05-06.
- ^ John Santarius (June 2006). "A Strategy for D – 3
He
Development" (PDF). Archived from the original (PDF) on 2007-07-03. Retrieved 2007-05-06. - ^ "Nuclear Reactions". Archived from the original on 2000-02-01. Retrieved 2007-05-06.
- ^ John Santarius (September 28, 2004). "Lunar 3
He
and Fusion Power" (PDF). Archived from the original (PDF) on 2007-07-03. Retrieved 2007-05-06. - ^ Mark Williams (August 23, 2007). "Mining the Moon: Lab experiments suggest that future fusion reactors could use helium-3 gathered from the moon". MIT Technology Review. Archived from the original on 2010-12-30. Retrieved 2011-01-25.
- ^ Date from the US Energy Information Administration
- ^ Paul DiMare (October 2004). "Mining The Moon". Popular Mechanics. Archived from the original on 2007-08-14. Retrieved 2007-05-06.
- ^ "ITER & Beyond". Archived from the original on 2009-05-20. Retrieved 2009-08-04.
- ^ "Helion FAQ". Retrieved 29 September 2022.
- ^ Todd Rider. "A general critique of inertial-electrostatic confinement fusion systems". hdl:1721.1/29869.
- ^ FTI Research Projects :: 3He Lunar Mining Archived 2006-09-04 at the Wayback Machine. Fti.neep.wisc.edu. Retrieved on 2011-11-08.
- ^ E. N. Slyuta; A. M. Abdrakhimov; E. M. Galimov (2007). "The estimation of helium-3 probable reserves in lunar regolith" (PDF). Lunar and Planetary Science XXXVIII (1338): 2175. Bibcode:2007LPI....38.2175S. Archived (PDF) from the original on 2008-07-05. Retrieved 2007-05-31.
- ^ Eric R. Hedman (January 16, 2006). "A fascinating hour with Gerald Kulcinski". The Space Review. Archived from the original on January 9, 2011. Retrieved August 30, 2007.
- ^ I.N. Sviatoslavsky (November 1993). "The challenge of mining He-3 on the lunar surface: how all the parts fit together" (PDF). Archived from the original (PDF) on 2019-01-20. Retrieved 2008-03-04. Wisconsin Center for Space Automation and Robotics Technical Report WCSAR-TR-AR3-9311-2.
- ^ "With He-3 on mind, India gets ready for lunar mission". The Times Of India. 2008-09-19. Archived from the original on 2008-09-21. Retrieved 2008-09-21.
- ^ Scientific Archived 2009-10-12 at the Wayback Machine. Isro.org (2008-11-11). Retrieved on 2011-11-08.
- ^ Luna C/I:: Chandrayaan-1 Payload Feature #2: Sub KeV Atom Reflecting Analyser (SARA) Archived 2019-07-20 at the Wayback Machine. Luna-ci.blogspot.com (2008-11-12). Retrieved on 2011-11-08.
- ^ He asked for the moon-and got it Archived 2023-06-15 at the Wayback Machine. Chinadaily.com.cn (2006-07-26). Retrieved on 2011-11-08.
- ^ Russian Rocket Builder Aims for Moon Base by 2015, Reports Say. Associated Press (via space.com). 26 January 2006
- ^ James Oberg (February 6, 2006). "Moonscam: Russians try to sell the Moon for foreign cash". Archived from the original on June 15, 2023. Retrieved August 30, 2007.
- ^ Dwayne A. Day (March 5, 2007). "Death throes and grand delusions". The Space Review. Archived from the original on June 15, 2023. Retrieved August 30, 2007.
- ^ Day, Dwayne (September 28, 2015). "The helium-3 incantation". The Space Review. Archived from the original on 27 December 2018. Retrieved 11 January 2019.
The belief in helium-3 mining is a great example of a myth that has been incorporated into the larger enthusiasm for human spaceflight, a magical incantation that is murmured, but rarely actually discussed.
- ^ Bryan Palaszewski. "Atmospheric Mining in the Outer Solar System" (PDF). Archived from the original (PDF) on 2009-03-27. NASA Technical Memorandum 2006-214122. AIAA–2005–4319. Prepared for the 41st Joint Propulsion Conference and Exhibit cosponsored by AIAA, ASME, SAE, and ASEE, Tucson, Arizona, July 10–13, 2005.
Bibliography
- Smith, D. M.; Goodwin, T. W.; Schillinger, J. A. (26 September 2003). "Challenges to the worldwide supply of helium in the next decade" (PDF). American Institute of Physics Conference Series. 49: 119–138. Bibcode:2004AIPC..710..119S. doi:10.1063/1.1774674. Archived (PDF) from the original on 2015-01-04. Retrieved 2024-09-10.
- L.J. Wittenberg (July 1994) [presented July 1993, revised July 1994]. Non-Lunar 3He Resources (PDF). Second Wisconsin Symposium on Helium-3 and Fusion Power. Archived (PDF) from the original on 2021-01-17. Retrieved 2024-09-10.
- H.H. Schmitt (2005). Return to the Moon: Exploration, Enterprise, and Energy in the Human Settlement of Space. Springer. ISBN 978-0-387-24285-9.
- J. Wilks (1967). The properties of liquid and solid helium. Oxford University Press.
- E. R. Dobbs (2000). Helium three. Oxford University Press.
- G. E. Volovik (1992). Exotic properties of superfluid 3He. World Scientific.
- W. P. Halperin, ed. (1990). Helium three. North-Holland.
- J. G. Daunt, ed. (1960). Helium three: proceedings of the Second Symposium on Liquid and Solid Helium Three, held at the Ohio State University, August 23--25, 1960. Ohio State University Press.
External links
- The Nobel Prize in Physics 2003, presentation speech Archived 2008-07-23 at the Wayback Machine
- Moon for Sale: A BBC Horizon documentary on the possibility of lunar mining of Helium-3 Archived 2023-06-15 at the Wayback Machine
