Group 14 hydride
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms (the elements of group 14 are carbon, silicon, germanium, tin, lead and flerovium).
Tetrahydrides
The tetrahydride series has the chemical formula XH4, with X representing any of the carbon family. Methane is commonly the result of the decomposition of organic matter and is a greenhouse gas. The other hydrides are generally unstable, poisonous metal hydrides.
They take on a pyramidal structure, and as such are not polar molecules like the other p-block hydrides.
Unlike other light hydrides such as ammonia, water and hydrogen fluoride, methane does not exhibit any anomalous effects attributed to hydrogen bonding, and so its properties conform well to the prevailing trend of heavier group 14 hydrides.
| Compound | Chemical formula | Molecular geometry | Space-filling model |
|---|---|---|---|
| carbon tetrahydride hydrogen carbide methane (carbane) |
CH4 |  |

|
| silicon tetrahydride hydrogen silicide (silane) |
SiH4 | 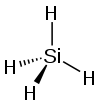 |

|
| germanium tetrahydride hydrogen germanide (germane) |
GeH4 |  |

|
| tin tetrahydride hydrogen stannide (stannane) |
SnH4 |  |

|
| lead tetrahydride hydrogen plumbide (plumbane) |
PbH4 | 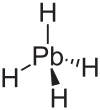 |

|
| flerovium tetrahydride hydrogen flerovide (flerovane) |
FlH4 |
Hexahydrides
This series has the chemical formula X2H6. Ethane is commonly found alongside methane in natural gas. The other hydrides of the chemical formula X2H6 are less stable than the corresponding tetrahydrides XH4, and they are more and more less stable as X goes from carbon (ethane C2H6 is stable) down to lead (or flerovium) in the periodic table (diplumbane Pb2H6 is unknown[1]).
| Compound | Chemical formula | Molecular geometry | Space-filling model |
|---|---|---|---|
| Ethane (dicarbon hexahydride) (dicarbane) |
C2H6 | 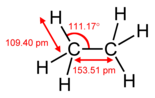 |

|
| Disilane (disilicon hexahydride) |
Si2H6 |  |

|
| Digermane (digermanium hexahydride) |
Ge2H6 |  |

|
| Distannane (ditin hexahydride) |
Sn2H6 |  |

|
| Diplumbane (dilead hexahydride) |
Pb2H6 |  |

|
| Diflerovane (diflerovium hexahydride) |
Fl2H6 |
Higher group 14 hydrides
All straight-chain saturated group 14 hydrides follow the formula XnH2n+2, the same formula for the alkanes.

Many other group 14 hydrides are known. Carbon forms a huge variety of hydrocarbons (among the simplest alkanes are methane CH4, ethane C2H6, propane C3H8, butane C4H10, pentane C5H12 and hexane C6H14, with a wide range of uses. There is also polyethylene (CH2)n, where n is very large, a stable hydrocarbon polymer, the most commonly produced plastic.[2] Hydrocarbons also include alkenes, which contain a double bond between carbon atoms (e.g. ethylene H2C=CH2), alkynes, which contain a triple bond between carbon atoms (e.g. acetylene H−C≡C−H), cyclic and branched hydrocarbons (e.g. cyclohexane C6H12, limonene C10H16, which is a cyclic hydrocarbon with double bonds between carbon atoms, and neopentane C(CH3)4, which is a branched hydrocarbon), as well as aromatic hydrocarbons such as benzene C6H6 and toluene C6H5−CH3), whose study forms the core of organic chemistry.[3]
Alongside hydrogen, carbon can form compounds with the chemically similar halogens, forming haloalkanes. The simplest of this series, the halomethanes, contain compounds such as dichloromethane CH2Cl2, chloroform CHCl3 and iodoform CHI3. Other such important chemicals include vinyl chloride H2C=CHCl, which is used in the production of PVC.
The other group 14 elements have a lower tendency to catenate. Hydrosilicons (binary silicon-hydrogen compounds), a silicon analogs of hydrocarbons, such as silanes SinH2n+2 are known for n = 1–8, in which thermal stability decreasing as n increases (e.g. silane SiH4 and disilane Si2H6), as are cyclosilanes (e.g. cyclopentasilane Si5H10 and cyclohexasilane Si6H12). They are very reactive, pyrophoric colourless gases or volatile liquids. Their volatility is intermediate between the alkanes and the germanes.[4] Unsaturated silanes, the silenes and silynes, have been characterized spectroscopically. The first members of each respectively are disilene H2Si=SiH2 and disilyne H−Si≡Si−H, the silicon analogues of ethylene and acetylene respectively.
The first five hydrogermaniums GenH2n+2 are known and are fairly similar to the hydrosilicones,[5] e.g. germane GeH4 and digermane Ge2H6. They are germanium analogues of alkanes.
Stannane SnH4, a strong reducing agent slowly decomposes at room temperature to tin and hydrogen gas, and is decomposed by concentrated aqueous acids or alkalis; distannane, Sn2H6 is still more unstable, and longer hydrostannums (hydrotins) are unknown. Stannane and distannane are tin analogues of methane and ethane respectively.
Plumbane PbH4 is very poorly characterised and is only known in trace amounts: even at low temperatures, synthesis methods that yield the other MH4 compounds fail to give PbH4. No other hydroplumbums (hydroleads) are known.[1] However, some substituted diplumbanes, with a general chemical formula R3Pb−PbR3 are more stable, where the R groups are organyl.
Compounds containing hydrogen and multiple group 14 elements are known, one of the most famous of these being tetraethyllead Pb(CH2CH3)4 which contains carbon and lead. The other examples are methylsilane H3C−SiH3 which contains carbon and silicon, tris(trimethylsilyl)germanium hydride ((CH3)3Si)3GeH which contain carbon, silicon and germanium, silylgermane or germylsilane H3Si−GeH3 which contains silicon and germanium, and hexaphenyldiplumbane (C6H5)3Pb−Pb(C6H5)3 which contains carbon and lead.[6]
See also
- Methylene CH2
- Methylidyne CH
- Titanium(IV) hydride TiH4, a structural analog of the group 14 tetrahydrides
- Zirconium hydride ZrH4, ZrH2 and others
- Zirconium(II) hydride ZrH2
- Uranium(IV) hydride UH4
References
- ^ a b Greenwood and Earnshaw, p. 375.
- ^ Whiteley, Kenneth S.; Heggs, T. Geoffrey; Koch, Hartmut; Mawer, Ralph L. and Immel, Wolfgang (2005) "Polyolefins" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi:10.1002/14356007.a21_487.
- ^ Greenwood and Earnshaw, p. 301.
- ^ Greenwood and Earnshaw, p. 337.
- ^ Greenwood and Earnshaw, p. 374.
- ^ "Hexaphenyldilead - Optional[1H NMR] - Spectrum - SpectraBase".
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.