Carboboration
In organic chemistry, carboboration describes an addition of both a carbon and a boron moiety to certain carbon-containing double and triple bonds, such as alkenes, alkynes, and allenes.
In the synthesis of organic compounds, this chemical reaction is used to install a new carbon-carbon bond and carbon-boron bond. The product of carboboration reactions are organoborane compounds which prove to be useful in organic synthesis, containing both a new carbon group and a boron handle for further functionalization. This carbon-boron bond allows for organoboron chemistry, which facilitates a wide variety of chemical transformations such as oxidation and the Suzuki Reaction. The carbon-boron bond can be transformed into a variety of functional groups and moieties, making it highly useful in pharmaceutical chemistry and organic synthesis.
Carboboration was developed soon after the advent and widespread use of hydroboration. Carboboration is often facilitated via catalysis, often employing transition metals, and usually involves an activated alkene or alkyne. The two most well-documented categories of carboboration are 1,1 and 1,2 carboboration, which differ in the regioselectivity of the incoming carbon group.
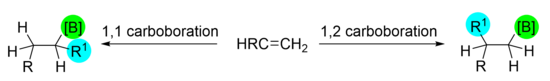
1,1 Carboboration
1,1 carboboration delivers both the carbon-carbon bond and the carbon-boron bond to the same carbon in the substrate. It requires a 1,2-migration of a substituent from one carbon to the other in the double bond. The Wrackmeyer reaction is typically credited as being the pioneering example of 1,1 carboboration and utilizes a metal migrating group to help facilitate the transformation.[1] However, there are several modern examples of carboboration with a variety of migrating groups.[2][3]
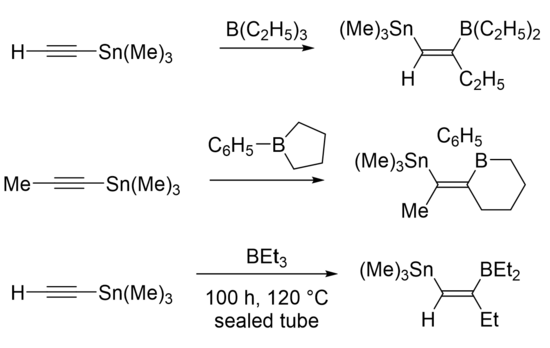
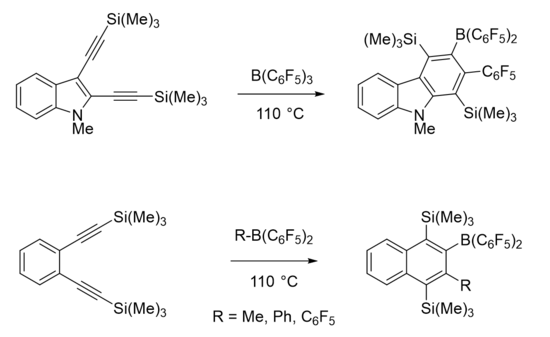
The Wrackmeyer reaction involves 1,1 carboboration of a 1-alkynylmetal compound to yield alkenylborane compounds. [M] can be silicon, germanium, tin, or lead compounds with various substituents or ligands. [M] and BR2 are typically cis to one another in the Wrackmeyer reaction, with some exceptions.[1]

Mechanism
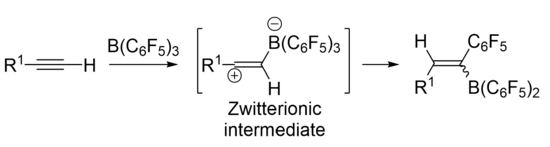
Wrackmeyer-type 1,1 carboboration is proposed to go through a zwitterionic intermediate, and this intermediate has been isolated and characterized in some cases.[4][5][6] However, the mechanism can be highly substrate and reagent dependent.
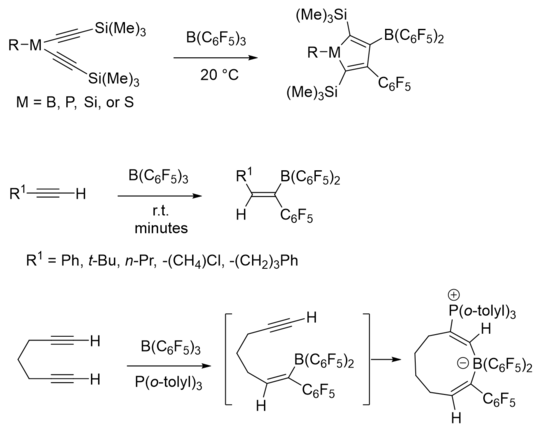
In a borane, the compound typically adopts a trigonal planar molecular geometry, making the boron atom an electrophilic center. The substituents can affect the strength of the borane as a Lewis acid.[5] Boranes which are stronger Lewis acids are better electrophiles and therefore better able to facilitate carboboration. Boranes can be optimized to work on less activated substrates. Tris(pentafluorophenyl)borane [B(C6F5)3] is a strongly Lewis acidic borane which functions well in 1,1 carboborations with both activated and unactivated substrates, and it allows for the reaction to be facilitated with more mild conditions.[3] An activated substrate such as an alkene or alkyne has an electron-withdrawing group directly attached to a carbon within the double or triple bond.[7] Transition metal catalysts have been utilized to develop enantioselective 1,1 carboborations on unactivated alkenes. These reactions go through a catalytic cycle which may or may not go through a zwitterionic intermediate.[8]
Examples


1,2 Carboboration
1,2 carboboration delivers the carbon-carbon bond and the carbon-boron bond to adjacent carbons in the substrate. It is typically facilitated by transition metal catalysis, but transition-metal-free 1,2 carboborations have been developed and continue to be of interest to synthetic chemists.[11] The benefit of utilizing transition metals is that the reactions can often have enantioselective control based on the ligands used on the metal complex. Common metals used are palladium, nickel, and copper, which are often coupled with an organoborane or a boron source with an electrophile or nucleophile.[12]

Mechanism
The mechanism of carboboration depends highly on the substrate and reagents utilized in the reaction. Shown below are examples of two types of Pd-catalyzed alkene 1,2 carboborations, Heck-type and the Wacker-type.[12] However, the Cu- and Ni-catalyzed reactions can proceed through similar mechanisms. These two mechanisms mainly differ in the oxidation state of the active catalyst and how the carbon group is delivered to the substrate: whether the C–C bond is formed via migratory insertion from the catalyst (inner sphere) or attack by an external nucleophile (outer sphere). Wacker-type carboborations, catalyzed by PdII, are much rarer than Heck-type. The first example of a Wacker-type 1,2 carboboration was reported by the Engle group in 2019.[13]
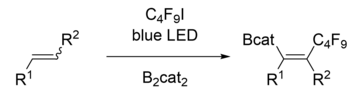
Despite the common trend of utilizing transition metals, transition metal-free processes have also been developed, such as utilizing boronic acids[11] or light-mediated radical initiation.[14] These reactions usually lead to the boron substituent being at the terminus or less substituted side of the substrate, but anti-carborborations have also been developed which produce reverse regioselectivity.[11][13][15] Much work has also been done to render 1,2 carboboration enantioselective using various ligands on transition metal catalysts.

Examples

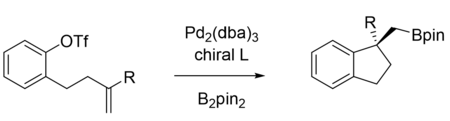
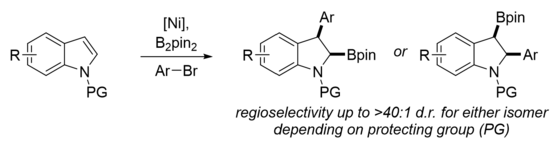


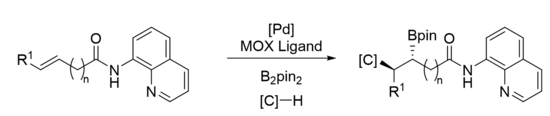


1,n Carboboration
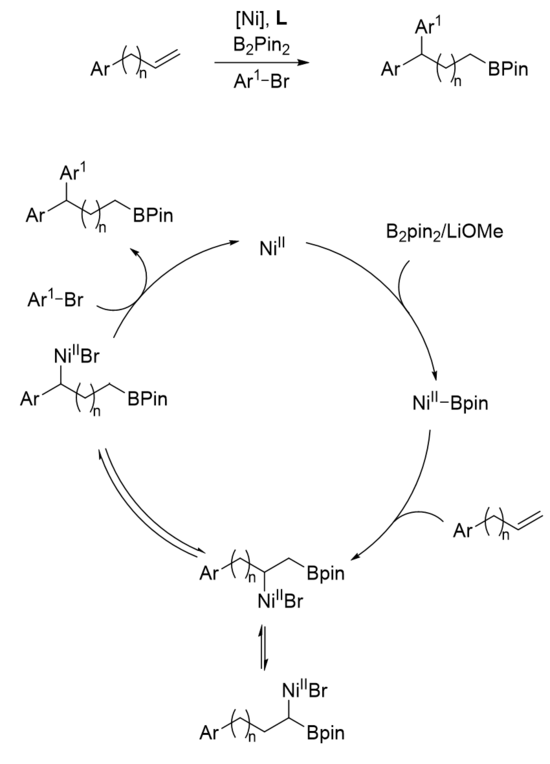
A nickel-catalyzed 1,n arylboration was developed in 2019 by Yin and coworkers and remains the only example of a chain-walking arylboration.[23] This was accomplished via a nitrogen-based ligand and a three-component coupling. The general scheme plus proposed mechanism is shown.
References
- ^ a b c Wrackmeyer, B. (1995). "l,l-Organoboration of alkynylsilicon, -germanium, -tin and -lead compounds". Coordination Chemistry Reviews. 145: 125.
- ^ a b Kehr G.; Erker, G. (2016). "Advanced 1,1-carboboration reactions with pentafluorophenylboranes". Chemical Science. 7 (1): 56–65. doi:10.1039/c5sc03282b. PMC 5508682. PMID 28757997.
- ^ a b Kehr G.; Erker, G. (2012). "1,1-Carboboration". Chemical Communications. 48 (13): 1839–1850. doi:10.1039/C1CC15628D. PMID 22116402.
- ^ a b Bismuto, A.; Thomas, S. P.; Duarte, F; Cowley, M. J. (2012). "Characterization of the Zwitterionic Intermediate in 1,1-Carboboration of Alkynes". Chemical Communications. 48 (13): 1839–1850. doi:10.1039/C1CC15628D. PMID 22116402.
- ^ a b c Wrackmeyer, B.; Khan, E. (2015). "1,1-Carboboration through Activation of Silicon–Carbon and Tin–Carbon Bonds". European Journal of Inorganic Chemistry. 2016 (3): 300. doi:10.1002/ejic.201500727.
- ^ Wrackmeyer, B.; Kehr, G.; Suß, J.; Molla, E. (1999). "1,1-Organoboration of tetraynes—routes to new siloles, stannoles and fused heterocycles". Journal of Organometallic Chemistry. 577: 82. doi:10.1016/S0022-328X(98)01029-8.
- ^ Costa, D. (2020). "Additions to non-activated alkenes: Recent advances". Arabian Journal of Chemistry. 13: 799. doi:10.1016/j.arabjc.2017.07.017. S2CID 102556829.
- ^ a b Wang, W.; Ding, C.; Yin, G. (2020). "Catalyst-controlled enantioselective 1,1-arylboration of unactivated olefins". Nature Catalysis. 3 (11): 951. doi:10.1038/s41929-020-00523-8. S2CID 224783863.
- ^ Wrackmeyer, B.; Menz, G. (1977). "Organoborierung von Alkinylstannanen, III1 Zur Reaktion von Trialkylboranen mit Monoalkinylstannanen / Organoboration of Alkynylstannanes, III The Reaction of Trialkylboranes with Monoalkynylstannanes". Zeitschrift für Naturforschung B (in German). 32b (12): 1400. doi:10.1515/znb-1977-1209. S2CID 98409890.
- ^ Sun, S. Z.; Talavera, L.; Spieß, P.; Day, C. S.; Martin, R. (2021). "sp3 Bis-Organometallic Reagents via Catalytic 1,1-Difunctionalization of Unactivated Olefins". Angewandte Chemie. 60 (21): 11740–11744. doi:10.1002/anie.202100810. PMID 33630396. S2CID 232050085.
- ^ a b c d Roscales, S.; Csákÿ, A. G. (2015). "Transition-Metal-Free Direct anti-Carboboration of Alkynes with Boronic Acids To Produce Alkenylheteroarenes". Organic Letters. 17 (6): 1605–1608. doi:10.1021/acs.orglett.5b00517. PMID 25738233.
- ^ a b c Liu, Z.; Gao, Y.; Zeng, T.; Engle, K. M. (2020). "Transition-Metal-Catalyzed 1,2-Carboboration of Alkenes: Strategies, Mechanisms, and Stereocontrol". Israel Journal of Chemistry. 60 (3–4): 219–229. doi:10.1002/ijch.201900087. PMC 8006804. PMID 33785969.
- ^ a b Liu, Z.; Ni, H. Q.; Zeng, T.; Engle, K. M. (2018). "Catalytic Carbo- and Aminoboration of Alkenyl Carbonyl Compounds via Five- and Six-Membered Palladacycles". Journal of the American Chemical Society. 140 (9): 3223–3227. doi:10.1021/jacs.8b00881. PMC 6002770. PMID 29384373.
- ^ a b Jin, S.; Larionov, O. V. (2018). "A Radical New Look for Alkene Carboboration". Chem. 4 (6): 1205. Bibcode:2018Chem....4.1205J. doi:10.1016/j.chempr.2018.05.022. S2CID 103226343.
- ^ Liu, Z.; Chen, J.; Lu, H. X.; Li, X.; Gao, Y.; Coombs, J. R.; Goldfogel, M. J.; Engle, K. M. (2019). "Pd(0)-Catalyzed Directed syn-1,2-Carboboration and -Silylation: Alkene Scope, Applications in Dearomatization, and Stereocontrol via a Chiral Auxiliary". Angewandte Chemie International Edition in English. 58 (47): 17068–17073. doi:10.1002/anie.201910304. PMC 7337986. PMID 31538388.
- ^ Ye, Y.; Lin, Y.; Mao, N. D.; Yang, H.; Ye, X. Y.; Xie, T. (2018). "Recent progress in nickel-catalyzed carboboration of alkenes". Organic & Biomolecular Chemistry. 140 (9): 3223–3227. doi:10.1021/jacs.8b00881. PMC 6002770. PMID 29384373.
- ^ Zieba, A.; Hooper, J. F. (2021). "Palladium-Catalysed Carboborylation for the Synthesis of Borylated Indanes". European Journal of Organic Chemistry. 2021 (29): 4072. doi:10.1002/ejoc.202100309. S2CID 234865746.
- ^ Trannel, G. L.; Kuniyil, R.; Crook, P. F.; Liu, P.; Brown, M. K. (2021). "Nickel-Catalyzed Dearomative Arylboration of Indoles: Regioselective Synthesis of C2- and C3-Borylated Indolines". Journal of the American Chemical Society. 143 (40): 16502–16511. doi:10.1021/jacs.1c05902. PMC 8781163. PMID 34582691.
- ^ Dorn, S. K.; Brown, M. K. (2022). "Cooperative Pd/Cu Catalysis for Alkene Arylboration: Opportunities for Divergent Reactivity". ACS Catalysis. 12 (3): 2058–2063. doi:10.1021/acscatal.1c05696. PMC 9540610. PMID 36212545.
- ^ Gao, Y.; Kim, N.; Mendoza, S. D.; Yazdani, S.; Vieira, A. F.; Liu, M.; Grotjahn, D. B.; Bertrand, G.; Jazzar, R.; Engle, K. M. (2022). "(CAAC)Copper Catalysis Enables Regioselective Three-Component Carboboration of Terminal Alkynes". ACS Catalysis. 12 (12): 7243–7247. doi:10.1021/acscatal.2c00614. PMC 10153597. PMID 37143933.
- ^ Liu, Z.; Li, X. Q.; Zeng, T.; Engle, K. M. (2019). "Directed, Palladium(II)-Catalyzed Enantioselective anti-Carboboration of Alkenyl Carbonyl Compounds". ACS Catalysis. 9 (4): 3260–3265. doi:10.1021/acscatal.9b00181. PMC 6889887. PMID 31799023.
- ^ Averdunk, A.; Hasenbeck, M.; Müller, T.; Becker, J.; Gellrich, U. (2022). "1,2-Carboboration of Arylallenes by In Situ Generated Alkenylboranes for the Synthesis of 1,4-Dienes". Chemistry - A European Journal. 28 (32): e202200470. doi:10.1002/chem.202200470. PMC 9325554. PMID 35348257.
- ^ a b Wang, W.; Ding, C.; Li, Y.; Li, Z.; Li, Y.; Peng, L.; Yin, G. (2019). "Migratory Arylboration of Unactivated Alkenes Enabled by Nickel Catalysis". Angewandte Chemie International Edition. 58 (14): 4612–4616. doi:10.1002/anie.201814572. PMID 30740847. S2CID 73450688.
