Barium iodide
| |
| Names | |
|---|---|
| IUPAC name Barium iodide | |
| Other names Barium iodide, anhydrous | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.873 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BaI2 (anhydrous) BaI2·2H2O (dihydrate) | |
| Molar mass | 391.136 g/mol (anhydrous) 427.167 g/mol (dihydrate) |
| Appearance | White orthorhombic crystals (anhydrous) colorless crystals (dihydrate) |
| Odor | odorless |
| Density | 5.15 g/cm3 (anhydrous) 4.916 g/cm3 (dihydrate) |
| Melting point | 711 °C (1,312 °F; 984 K) (anhydrous) decomposes at 740 °C (dihydrate) |
| 166.7 g/100 mL (0 °C) 221 g/100 mL (20 °C) 246.6 g/100 mL (70 °C) | |
| Solubility | soluble in ethanol, acetone |
| -124.0·10−6 cm3/mol | |
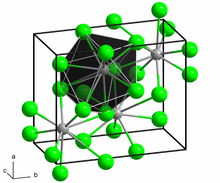
| Structure | |
| PbCl2-type (Orthorhombic oP12) | |
| Pnma (No. 62) | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-602.1 kJ·mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
toxic |
| Related compounds | |
Other anions |
barium fluoride barium chloride barium bromide |
Other cations |
beryllium iodide magnesium iodide calcium iodide strontium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Barium iodide is an inorganic compound with the formula BaI2. The compound exists as an anhydrous and a hydrate (BaI2(H2O)2), both of which are white solids. When heated, hydrated barium iodide converts to the anhydrous salt. The hydrated form is freely soluble in water, ethanol, and acetone.
Structure
The structure of the anhydrous form resembles that of lead(II) chloride with each Ba center bound to nine iodide ligands[2] and has a crystalline packing structure that is quite similar to BaCl2.[3]
Reactions
Anhydrous BaI2 can be prepared by treating Ba metal with 1,2-diiodoethane in ether.[4]
BaI2 reacts with alkyl potassium compounds to form organobarium compounds.[5]
BaI2 can be reduced with lithium biphenyl, to give a highly active form of barium metal.[6]
Safety
Like other soluble salts of barium, barium iodide is toxic.
References
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–44, ISBN 0-8493-0594-2
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Brackett, E. B.; Brackett, T. E.; Sass, R. L.; The Crystal Structures of Barium Chloride, Barium Bromide, and Barium Iodide. J. Phys. Chem., 1963, volume 67, 2132 – 2135
- ^ Duval, E.; Zoltobroda, G.; Langlois, Y.; A new preparation of BaI2: application to (Z)-enol ether synthesis. Tetrahedron Letters, 2000, 41, 337-339
- ^ Walter, M. D.; Wolmershauser, G.; Sitzmann, H.; Calcium, Strontium, Barium, and Ytterbium Complexes with Cyclooctatetraenyl or Cyclononatetraenyl Ligands. J. Am. Chem. Soc., 2005, 127 (49), 17494 – 17503.
- ^ Yanagisawa, A.; Habaue, S.; Yasue, K.; Yamamoto, H.; Allylbarium Reagents: Unprecedented Regio- and Stereoselective Allylation Reactions of Carbonyl Compounds. J. Am. Chem. Soc.1994, 116,6130-6141
