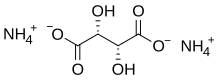
Ammonium tartrate
 | |
| Names | |
|---|---|
| IUPAC name diazanium;(2R,3R)-2,3-dihydroxybutanedioate | |
| Other names L-(+)-Tartaric acid diammonium salt, Diammonium tartrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.654 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H12N2O6 | |
| Molar mass | 184.148 g·mol−1 |
| Appearance | colorless crystals |
| Density | 1.601 g/cm3 |
| Boiling point | 399.3 °C |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Ammonium tartrate is a chemical compound with the chemical formula (NH4)2C4H4O6.[1][2] This is an organic ammonium salt of tartaric acid.
Synthesis
Ammonium tartrate can be prepared by the reaction of tartaric acid and ammonium carbonate.
Physical properties
Ammonium tartrate forms colorless crystals that slowly release ammonia if exposed to air.[3] Easily soluble in water,[4] also soluble in alcohol.[5]
Ammonium tartrate crystallizes in the monoclinic crystal system with the space group P21 (space group No. 4) with the lattice parameters a = 708 pm, b = 612 pm, c = 880 pm, β = 92.42 ° and Z = 2.[6]
Uses
The compound is used in textile industry and in medicine.[3][7]
See also
References
- ^ Report of the ... Meeting. Murray. 1896. p. 254. Retrieved 5 March 2025.
- ^ "Ammonium tartrate dibasic". Sigma Aldrich. Retrieved 5 March 2025.
- ^ a b "Ammonium Tartrate" (PDF). New Jersey Department of Health. Retrieved 5 March 2025.
- ^ "Ammoniumtartrat" (in German). gestis.dguv.de. Retrieved 5 March 2025.
- ^ The Chemical News: With which is Incorporated the Chemical Gazette: a Journal of Practical Chemistry in All Its Applications to Pharmacy, Arts, and Manufactures. C. Mitchell and Company. 1870. p. 18. Retrieved 5 March 2025.
- ^ Yadava, V. S.; Padmanabhan, V. M. (15 March 1973). "The crystal structure of ammonium tartrate". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 29 (3): 493–498. Bibcode:1973AcCrB..29..493Y. doi:10.1107/S0567740873002803. ISSN 0567-7408. Retrieved 5 March 2025.
- ^ Pohanish, Richard P. (4 November 2011). Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens. William Andrew. p. 207. ISBN 978-1-4377-7869-4. Retrieved 5 March 2025.
