2C-T-8
| |

| |
| Names | |
|---|---|
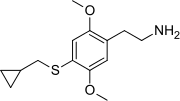
| Preferred IUPAC name 2-{4-[(Cyclopropylmethyl)sulfanyl]-2,5-dimethoxyphenyl}ethan-1-amine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H21NO2S | |
| Molar mass | 267.39 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
2C-T-8 is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin, sometimes used as an entheogen.
Chemistry
The full name of the chemical is 2,5-dimethoxy-4-cyclopropylmethylthiophenethylamine. The compound is reported to have a bad taste and smell.[citation needed]
Effects
In his book PiHKAL, Shulgin lists the dosage range as 30 to 50 mg.[1] 2C-T-8 is generally taken orally and effects typically last 10 to 15 hours. Experiences have varied between insight and creativity at low doses to hypersensitivity and paranoia at higher doses. A "thinking-connection" that is characteristic of the 2C-T group is evident in this chemical in stark contrast to the "pure euphoria" of phenethylamines such as MDMA.[1]
Legality
2C-T-8 is unscheduled and uncontrolled in the United States, but possession and sales of 2C-T-8 will probably be prosecuted under the Federal Analog Act because of its structural similarities to 2C-T-7. However, 2C-T-8, unlike many other phenethylamines has not been sold by internet retailers. In the wake of Operation Web Tryp in July 2004, the issue of possession and sales of 2C-T-8 and other similar chemicals will probably be resolved in the courtroom as will the fate of this rare but unique psychedelic.[needs update]
Canada
As of October 31, 2016, 2C-T-8 is a controlled substance (Schedule III) in Canada.[2]
Pharmacology
The mechanism that produces 2C-T-8's hallucinogenic and entheogenic effects has not been specifically established, however it is most likely to result from action as a 5-HT2A serotonin receptor agonist in the brain, a mechanism of action shared by all of the hallucinogenic tryptamines and phenethylamines for which the mechanism of action is known.
Dangers
The toxicity of 2C-T-8 is not well documented. 2C-T-8 is somewhat less potent than 2C-T-7, but it may be expected that at higher doses it would display similar toxicity to that of other phenethylamines of the 2C-T family.
There have been no confirmed deaths due to 2C-T-8,[citation needed] though this may in part be due to its rarity and limited usage. Of the 2C-T family, there have been a few confirmed deaths due to 2C-T-7, which involved either insufflating large (>30 mg) doses[3][4] and in one case an unknown oral dose was combined with 200 mg MDMA.[5]
Popularity
2C-T-8 is unknown on the black market. Limited accounts of 2C-T-8 can be found in the book PiHKAL.
References
- ^ a b Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. 2C-T-8 Entry in PiHKAL
- ^ "Canada Gazette – Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". 4 May 2016.
- ^ Erowid.org
- ^ Erowid.org
- ^ Erowid.org
