17α-Hydroxyprogesterone

| |

| |
| Names | |
|---|---|
| IUPAC name 17α-Hydroxypregn-4-ene-3,20-dione | |
| Systematic IUPAC name (1R,3aS,3bR,9aR,9bS,11aS)-1-Acetyl-1-hydroxy-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names Hydroxyprogesterone (INN) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.636 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H30O3 | |
| Molar mass | 330.46 g/mol |
| Melting point | 219.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP),[1] or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone.[2][3][4] It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.
Biological activity
17α-OHP is an agonist of the progesterone receptor (PR) similarly to progesterone, albeit weakly in comparison.[5] In addition, it is an antagonist of the mineralocorticoid receptor (MR)[6] as well as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol) at the latter site, also similarly to progesterone.[5][7][8]
| Compound | hPR-A | hPR-B | rbPR | rbGR | rbER | |||
|---|---|---|---|---|---|---|---|---|
| Progesterone | 100 | 100 | 100 | <1 | <1 | |||
| 17α-Hydroxyprogesterone | 1 | 1 | 3 | 1 | <1 | |||
| Hydroxyprogesterone caproate | 26 | 30 | 28 | 4 | <1 | |||
| Hydroxyprogesterone acetate | 38 | 46 | 115 | 3 | ? | |||
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, dexamethasone for the GR, and estradiol for the ER. Sources: See template. | ||||||||
Biochemistry
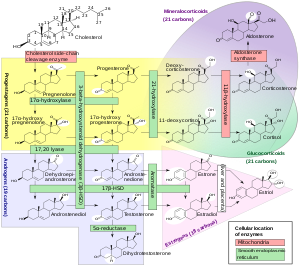
Biosynthesis
17α-OHP is derived from progesterone via 17α-hydroxylase (encoded by CYP17A1).[9]
17α-OHP increases in the third trimester of pregnancy primarily due to fetal adrenal production.[10]
This steroid is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 20-100 ng/dl prior to ovulation, and 100-500 ng/dl during the luteal phase.[11][12]
Measurement
Measurements of levels of 17α-OHP are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17α-OHP.[13] In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17α-OHP.[9] 17α-OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but note, 17α-OHP is also contributed by the placenta.[14]
Immunoassays like RIA (radioimmunoassay) or IRMA (immunoradiometric assay) used to clinically determine 17α-OHP are prone to cross-reactivity with the 17α-OHP steroid precursors and their sulphated conjugates. Gas or liquid chromatography and mass spectrometry (e.g. LC-MS/MS) achieves greater specificity than immunoassays.[15][16]
Measurement of 17α-OHP by LC-MS/MS improves newborn screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency, because 17α-OHP steroid precursors and their sulphated conjugates which are present in the first two days after birth and longer in pre-term neonates, cross-react in immunoassays with 17α-OHP, giving falsely high 17α-OHP levels.[15][16]
Pharmacology
Pharmacokinetics
Although 17α-OHP has not been used as a medication, its pharmacokinetics have been studied and reviewed.[17]
Medical uses
Esters of 17α-OHP, such as hydroxyprogesterone caproate and, to a far lesser extent, hydroxyprogesterone acetate and hydroxyprogesterone heptanoate, have been used in medicine as progestins.[2][3][4]
Chemistry
17α-OHP is the parent compound of a class of progestins referred to as the 17α-hydroxyprogesterone derivatives.[18][19][20] Among others, this class of drugs includes chlormadinone acetate, cyproterone acetate, hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate.[18][19][20]
Society and culture
Generic names
Hydroxyprogesterone is the generic name of 17α-OHP and its INN and BAN.[2][3][4]
See also
- 11α-Hydroxyprogesterone
- 5α-Dihydroprogesterone
- 20-Dihydroprogesterone
- 11-Deoxycorticosterone
- 11-Deoxycortisol
- 17α-Methylprogesterone
- 19-Norprogesterone
- 19-Nortestosterone
References
- ^ "17-hydroxyprogesterone (17OHP)".
- ^ a b c J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 664–665. ISBN 978-1-4757-2085-3.
- ^ a b c I.K. Morton, Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 146–. ISBN 978-94-011-4439-1.
- ^ a b c Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1.
- ^ a b Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032. PMID 18060946.
- ^ Mooij CF, Parajes S, Pijnenburg-Kleizen KJ, Arlt W, Krone N, Claahsen-van der Grinten HL (April 2015). "Influence of 17-Hydroxyprogesterone, Progesterone and Sex Steroids on Mineralocorticoid Receptor Transactivation in Congenital Adrenal Hyperplasia" (PDF). Horm Res Paediatr. 83 (6): 414–421. doi:10.1159/000374112. PMID 25896481. S2CID 24727940.
- ^ Pijnenburg-Kleizen KJ, Engels M, Mooij CF, Griffin A, Krone N, Span PN, van Herwaarden AE, Sweep FC, Claahsen-van der Grinten HL (2015). "Adrenal Steroid Metabolites Accumulating in Congenital Adrenal Hyperplasia lead to Transactivation of the Glucocorticoid Receptor". Endocrinology. 156 (10): 3504–3510. doi:10.1210/en.2015-1087. PMID 26207344.
- ^ Sun K, Lei K, Chen L, Georgiou EX, Sooranna SR, Khanjani S, Brosens JJ, Bennett PR, Johnson MR (2012). "Progesterone Acts via the Nuclear Glucocorticoid Receptor to Suppress IL-1β-Induced COX-2 Expression in Human Term Myometrial Cells". PLOS ONE. 7 (11): e50167. Bibcode:2012PLoSO...750167L. doi:10.1371/journal.pone.0050167. ISSN 1932-6203. PMC 3509141. PMID 23209664.
- ^ a b Kim SM, Rhee JH (2015). "A case of 17 alpha-hydroxylase deficiency". Clinical and Experimental Reproductive Medicine. 42 (2). The Korean Society for Reproductive Medicine: 72–76. doi:10.5653/cerm.2015.42.2.72. ISSN 2233-8233. PMC 4496435. PMID 26161337.
- ^ Tal R, Taylor HS (2021-03-18). "Endocrinology of Pregnancy". MDText.com, Inc. PMID 25905197. Retrieved 2024-06-25.
- ^ Reference Values During Pregnancy
- ^ "normal ranges for hormone tests in women". Archived from the original on 2020-11-08. Retrieved 2011-08-07.
- ^ Held PK, Bird IM, Heather NL (2020-08-23). "Newborn Screening for Congenital Adrenal Hyperplasia: Review of Factors Affecting Screening Accuracy". International Journal of Neonatal Screening. 6 (3). MDPI AG: 67. doi:10.3390/ijns6030067. ISSN 2409-515X. PMC 7569755. PMID 33117906.
- ^ Check JH, Vaze MM, Epstein R, Wu CH, Quattrocchi J, Vetter B (1990). "17-Hydroxyprogesterone level as a marker for corpus luteum function in aborters versus nonaborters". International Journal of Fertility. 35 (2): 112–115. ISSN 0020-725X. PMID 1970979.
- ^ a b de Hora MR, Heather NL, Patel T, Bresnahan LG, Webster D, Hofman PL (March 2020). "Measurement of 17-Hydroxyprogesterone by LCMSMS Improves Newborn Screening for CAH Due to 21-Hydroxylase Deficiency in New Zealand". International Journal of Neonatal Screening. 6 (1): 6. doi:10.3390/ijns6010006. PMC 7422986. PMID 33073005.
- ^ a b Bialk ER, Lasarev MR, Held PK (September 2019). "Wisconsin's Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia". International Journal of Neonatal Screening. 5 (3): 33. doi:10.3390/ijns5030033. PMC 7510207. PMID 33072992.
- ^ Die Gestagene. Springer-Verlag. 27 November 2013. pp. 276–277. ISBN 978-3-642-99941-3.
- ^ a b Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 289–. ISBN 978-0-08-093292-7.
- ^ a b Robert Alan Prentky, Ann Wolbert Burgess (31 July 2000). Forensic Management of Sexual Offenders. Springer Science & Business Media. pp. 219–. ISBN 978-0-306-46278-8.
- ^ a b H. J. Smith, Hywel Williams (1 January 1983). Introduction to the Principles of Drug Design. Elsevier. pp. 187–. ISBN 978-1-4831-8350-3.
